
Extracorporeal membrane oxygenation treatment of fulminant myocarditis: the key role of point-of-care ultrasound in pacemaker lead perforation
Introduction
Fulminant myocarditis (FM) is one of the key causes of arrhythmia, an uncommon syndrome characterized by sudden and severe diffuse cardiac inflammation. FM can also lead to cardiogenic shock, multi-organ system failure, and in severe cases, even death. All patients with FM require some form of inotropic or mechanical circulatory support to maintain end-to-end fusion until transportation or recovery (1). Pacemakers are widely used for the management of cardiac arrhythmias, but there are also several complications, such as infection, wire dislocation or displacement, and pacing function, that can lead to perforation in severe cases. Pacemaker lead–related myocardial perforation refers to a complication caused by various factors during permanent or temporary pacemaker implantation, in which the electrode lead penetrates the myocardium into the pericardial cavity or even the pleural cavity and results in cardiac tamponade. Studies have reported overall lead perforation rates after pacemaker implantation to be between 0.1% and 0.8% (2,3), with most perforations caused by right ventricle (RV) lead perforation of the apex. The temporary pacemaker, an active-fixation lead, and advanced age have been reported to be the risk factors of myocardial perforation (4).
Case presentation
A 74-year-old man with a medical history of hypertension presented with reduced appetite and a 2-day history of paroxysmal chest tightness lasting several hours at a time without obvious inducement. The patient also had dizziness on the day of admission and a self-measured blood pressure of 83/52 mmHg. After the patient fainted twice at a local hospital, an electrocardiograph (ECG) examination indicated myocardial infarction. After referral to the study hospital, the patient developed recurrent syncope and undetectable blood pressure. An ECG examination indicated third-degree atrioventricular block, the heart rate was about 30 beats per minute (bpm), and a subsequent ventricular fibrillation attack occurred. All procedures performed in this article were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
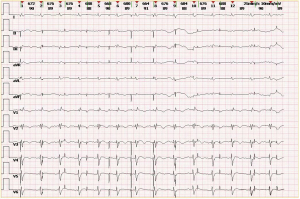
The patient underwent a procedure for the placement of a temporary active fixation ventricular pacemaker. Ultrasound examinations were performed by doctors from the department of ultrasound medicine. Acute myocardial infarction was ruled out via coronary angiography, and point-of-care transthoracic echocardiography (TTE) and ventriculography indicated weakening of whole-heart fluctuations. Although the patient had no obvious symptoms of infection, fever, or diarrhea in the month prior to admission, a diagnosis of FM was confirmed. On the second day, the patient’s condition was complicated by respiratory failure, and the frequent occurrence of ventricular fibrillation and ventricular tachycardia. The patient had unstable oxygen saturation, progressive increase of lactic acid, insufficient blood and oxygen supply to peripheral organs, and poor cardiac function. Although he had been assisted by tracheal intubation and ventilator, due to severe myocarditis and malignant arrhythmias such as frequent ventricular premature beats of life-threatening arrhythmias, and he underwent venoarterial extracorporeal membrane oxygenation (VA ECMO) treatment with point-of-care ultrasound (POCUS) assistance and was subsequently admitted to the intensive care unit for further treatment. Four days after VA ECMO implantation, TTE revealed a left ventricular ejection fraction of 30% to 40% with an overall decrease of wall motion and a large pericardial effusion (PE). Further examination indicated that the temporary pacemaker had migrated into the RV outflow tract (RVOT) and penetrated the RV wall (Figure 1A-1C; Videos 1,2). POCUS was performed throughout the course of the case diagnosis and treatment. The results of the ECG examination performed on the same day are shown in Figure 2. Comparing point-of-care chest X-ray (CXR) from the day of pacemaker implantation against those obtained on the third day post-surgery showed pacemaker electrode migration and cardiac shadow changes (Figure 3A,3B).


Four days after VA ECMO implantation, laboratory examinations at the time revealed a troponin I level decrease from 70.341 to 14.836 µg/L. The patient’s pulsatile blood pressure dropped at 8 PM. Vital signs of hypoxia were significant as follows, the heart rate was about 100 to 110 bpm, and the right radial artery had an invasive pulsatile blood pressure fluctuation of 80–95/40–50 mmHg. Cardiac auscultation detected low and distant heart sounds. The pulse of the patient’s dorsalis pedis artery was significantly reduced.
Due to the patient’s particular condition, multidisciplinary consultation was carried out. It was decided that catheter drainage of PE should be performed first and that two options for the treatment of pacemaker guidewire perforation could be considered. Given the lack of hardware in our hospital, such as quantitative expert TEE guidance or a hybrid operating room under VA ECMO support, the first option was to remove the temporary pacemaker directly under POCUS monitoring and closely monitor for active bleeding, which if present, would require urgent revision of the thoracic surgery. The second option was to perform surgical repair directly, but this entails higher risks for severe patients. After comprehensive consideration, the first option was ultimately selected. The patient tolerated extraction of the pacemaker electrode lead without complications, his condition improved, and he ultimately survived.
The lead was removed under the guidance of point-of-care TTE without the need for surgical intervention. The patient was weaned off VA ECMO 5 days later, and a reexamination of point-of-care TTE 6 days later showed no PE.
Discussion
POCUS played a critical role throughout the diagnosis and treatment process in this case, including in assessing the function of various organs. It was especially valuable when VA ECMO catheter insertion was performed under the guidance of ultrasound, and then the location of the pacemaker lead was discovered and changes in PE monitored. TTE can be used to identify and locate PE and guide pericardiocentesis (5). As a nonionizing radiation method, POCUS can be repeated without harming the patient. Naturally, there are some limitations to POCUS. For one, ultrasonic diagnosis depends on the experience and diagnostic level of the operator. In addition, POCUS is greatly affected by the surrounding tissue structure. For example, the images of patients with obesity or emphysema are not often clear, so it is necessary to combine POCUS with CXR and computerized tomography (CT) when necessary. CT is the gold standard diagnostic tool; however, in our case, the patient had severe complications, and thus POCUS was used to avoid the risks associated with CT examination during transport. POCUS imaging is useful for the rapid detection of PE, ascertaining sonographic evidence of tamponade sign, and searching for a displaced pacing lead for rapid diagnosis (6).
Although CXR may be the initial diagnostic tool of choice, it may not be able to detect minimal lead migration. Perforation is difficult to diagnose on chest radiography, and although CT remains the gold standard diagnostic tool for precisely demonstrating the pacemaker lead position, POCUS is a timely, efficient, effective, and patient-centered alternative in the emergency department setting (6). Our case was consistent with the findings in this literature. However, during ECMO, POCUS was used to detect the lead perforation in the anticoagulant state and to assess the dynamic risk of the condition, and we found no similar situation reported in the literature.
ECMO is used to treat patients with severe, life-threatening conditions of the heart and lungs. Anticoagulants are used to prevent clots from forming in the tubing that carries the blood; thus, bleeding is the most common complication of ECMO (7,8). During ECMO, anticoagulation is required to prevent thrombotic complications, but this increases the risk of pericardial puncture bleeding. In our case, ultrasound-guided pericardiocentesis procedure was performed and provided an excellent outcome. Because the intercostal puncture point was selected, the intercostal arteries were avoided, and the changes of PE could be dynamically observed during the puncture. This case demonstrates the critical value of POCUS in the early stages of diagnosis and the subsequent management of cardiac tamponade caused by pacemaker lead perforation. This is especially true when the patient is in an anticoagulant state after ECMO implantation.
Conclusions
In this case, except for the perforation of the pacemaker electrode lead under the special conditions of ECMO, conservative treatment was adopted to remove the pacemaker electrode lead after consultation with a multidisciplinary team. POCUS played a significant role in monitoring this process; for example, POCUS provided guidance for the formulation of the clinical treatment strategy, the removal of the pacemaker electrode lead in an anticoagulant state, the monitoring of the change in the PE amount after the removal of the pacemaker electrode lead, and the assessing of blood volume changes. POCUS has accumulated experience in the diagnosis of the disease, which may also provide new clues for the clinical treatment of the disease.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1386/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this article were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, Shah RV, Sims DB, Thiene G, Vardeny OAmerican Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation 2020;141:e69-92. [Crossref] [PubMed]
- Banaszewski M, Stępińska J. Right heart perforation by pacemaker leads. Arch Med Sci 2012;8:11-3. [Crossref] [PubMed]
- Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007;30:28-32. [Crossref] [PubMed]
- Frausing MHJP, Kronborg MB, Nielsen JC. Cardiac perforations by pacemaker and defibrillator leads: rare complications with severe implications. Europace 2022;24:1718-20. [Crossref] [PubMed]
- Caiati C, Pollice P, Truncellito L, Lepera ME, Favale S. Minimal Cardiac Perforation by Lead Pacemaker Complicated with Pericardial Effusion and Impending Tamponade: Optimal Management with No Pericardiocentesis Driven by Echocardiography. Diagnostics (Basel) 2020;10:191. [Crossref] [PubMed]
- Chen CC, Ho SW. Usage of point-of-care Ultrasonography for Rapid Diagnosis of Cardiac Perforation by Pacemaker Lead. J Med Ultrasound 2022;30:221-2. [Crossref] [PubMed]
- Hou X. Anticoagulation monitoring in extracorporeal membrane oxygenation. Perfusion 2021;36:438-9. [Crossref] [PubMed]
- Hadaya J, Benharash P. Extracorporeal Membrane Oxygenation. JAMA 2020;323:2536. [Crossref] [PubMed]


