Magnetic resonance imaging features of endometrial stromal sarcoma: a case description
Introduction
Endometrial stromal sarcoma (ESS) originates from mesenchyma or naive mesenchyma, accounting for only 0.2% of all uterine malignancies and 10–15% of uterus sarcoma. The annual incidence rate is 1–2 per million and the average age is 40–45 years old (1,2). ESS has a more preferable prognosis when compared with the more common endometrial carcinomas of epithelial origin. In 2003, ESS was classified into endometrial stromal nodule, low-grade endometrial stromal sarcoma (LGESS), high-grade ESS, undifferentiation endometrial stromal sarcoma (UES) by WHO. Early detection is necessary so to increase survive rate. Generally on magnetic resonance imaging (MRI) ESS shows the uterine body is enlarged with a well-circumscribed mass of inhomogeneous signals. In some circumstance, ESS presents hyperintensity on T2-weighted images, and isointensity relative to the myometrium on T1-weighted images. Moreover, obvious hyperintensity was observed on diffusion weighted imaging (3-5). Interestingly, in some cases, ESS may have a characteristic low-intensity rim on T2-weighted images (6). We herein present a case of a LGESS.
Case presentation
A 46-year-old woman was referred to our hospital on 12 March 2016. The patient developed hypogastralgia with no clear cause, simultaneously had frequent micturition and urgency of urination 8 days ago and gradually developed abdominal pain, chills and fever. This patient had normal menstrual cycle and excessive menstrual blood volume, meanwhile without dysmenorrhea. She had her last menstrual on 12 March 2016 with more than twice blood volume compared with normal and also much more blood clot than before. Local hospital reported a uterus masses after pelvic cavity ultrasonography.
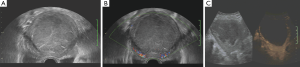
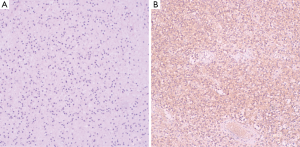
She was admitted to the department of gynaecology and obstetrics. Routine blood test found moderate anemia. Transvaginal ultrasonography (Voluson E8; GE Medical Systems, USA) showed a hypoechoic mass of to 96 mm × 78 mm in the uterine (Figure 1A), while the myometrium showed homogeneous echo with normal bloodstream signal (Figure 1B). Ultrasound angiography showed slight enhancement of capsule and no enhancement of lesion (Figure 1C). MRI (3.0 T, Discovery MR 750; GE Medical Systems, Milwaukee, WI, USA) showed that the uterine body was enlarged and a 7.8 cm × 8.8 cm × 9.9 cm abnormal mass with distinct margin in the uterine cavity with slight hypointensity on the T1-weighted image (Figure 2A) and slight hyperintensity on the T2-weighted image with mottled isointensity inside (Figure 2B). But it is worth that the signal on T1-weighted image of the mass higher than that of the urine and similar or higher than urine on the T2-weighted image. The mass have slight homogeneous enhancement after the injection of Gd-DTPA, and the edge of the mass has obvious enhancement, while the uterine wall has mild enhancement (Figure 2C,D). This woman underwent transabdominal total hysterectomy, bilateral complete salpingectomy. The tumor per se measured 11×10×12 cm3 and filled the uterine cavity. The mass was soft but solid, and reddish brown in color. Histological examination revealed that the tumor presented general degeneration and necrosis, nucleus karyopyknosis and sporadic arteriole (Figure 3A). Immunohistochemistry showed tumor cells were positive for CD10, Vimentin, ER and ki-67 (10%), in contrast, negative for MPO, LCA, inhibin, PR, Syn, CD20, CD3, SMA, and CgA (Figure 3B). This tumor was confirmed to be a LGESS.

Discussion
ESS is a rare malignant tumor originated from endometrial stromal cells with the characteristics of local infiltration, vascular invasion and frequent relapse (1). ESS is composed of four types. However, it is extremely difficult to make a diagnosis before surgery, as the imaging characteristic of this rare tumor type has not been established, especially for LGESS and UES (7). Both of them are histologically malignant, while the former have a fairly indolent course, the latter is fatal. The tumorigenesis of these tumors remains unknown. Experimental investigation shows that exposure to tamoxifen and unopposed estrogens has been implicated (8). A variety of morphologic appearances, including epithelial differentiation, a sex cord-like pattern, smooth muscle differentiation, and fibrous myxoid, have been reported (9). ESS is a hormone dependent and slow-growing disease which has a distinct boundary with the muscular layer and rich in blood vessels. Hemorrhage, necrosis and cystoids were frequently seen (10). Woman with ESS has median age between 40 and 45 years. The most common symptoms are abnormal vaginal bleeding, progressive menorrhagia, abdominal pain, and a pelvic mass (11). More than half the cases occur in premenopausal women, particularly those with LGESS (12).
Commonly ESS is divided into solid, cystic solid and cystic mass. According to the previous reports, the signal of the mass exhibits characteristic of inhomogeneous which presenting hyperintensity on T2-weighted images, and isointensity on T1-weighted images (4,13,14). However, in this case report, we can draw our attention that this tumor showed a mass which is solid but looked like being cystic on imaging, which is quite a rare. The signal on T1-weighted image of the mass showed slight hypointensity and slight hyperintensity with mottled isointensity on the T2-weighted image. This can help radiologists to identify it with other uterine cystic lesion. In the clinical practice radiologists should differentiate the ESS form the following diseases: (I) hematoma of myometrium, which presents isointensity or slightly hyperintensity on T1-weighted image, hyperintensity on T2-weighted image, and hyperintensity in diffusion weighted imaging, and no enhancement after contrast injection; (II) cystic degeneration of fibroid, which is tightly involved in myometrium and presents isointensity or slighted hypointensity on T1-weighted image and slight hyperintensity on T2-weighted image along with slighted or none enhancement (15). Beyond that, it also should be differentiated with the huge lesions derived from adnexal mass; (III) leiomyosarcoma, which has variable signal intensities on T2-weighted images and irregular margins of lesion and is difficult to differentiate with ESS even by MRI (16); (IV) endometrial cancers, which present low signal intensity on T1-weighted images and low signal intensity on T2-weighted images, on the contrary ESS is higher on T2-weighted images (17). Therefore, encountering with enormous lesions that looks like cystic mass in uterine, radiologists should analysis their imaging characteristic carefully and take into account the possibility of LGESS (18).
Acknowledgements
We gratefully acknowledge all authors cooperation in this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Puliyath G, Nair VR, Singh S. Endometrial stromal sarcoma. Indian J Med Paediatr Oncol 2010;31:21-3. [Crossref] [PubMed]
- Xue WC, Cheung AN. Endometrial stromal sarcoma of uterus. Best Pract Res Clin Obstet Gynaecol 2011;25:719-32. [Crossref] [PubMed]
- Hayasaka K, Morita K, Saitoh T, Tanaka Y. Uterine adenofibroma and endometrial stromal sarcoma associated with tamoxifen therapy: MR findings. Comput Med Imaging Graph 2006;30:315-8. [Crossref] [PubMed]
- Gandolfo N, Gandolfo NG, Serafini G, Martinoli C. Endometrial stromal sarcoma of the uterus: MR and US findings. Eur Radiol 2000;10:776-9. [Crossref] [PubMed]
- Toprak U, Paşaoğlu E, Karademir MA, Gülbay M. Sonographic, CT, and MRI findings of endometrial stromal sarcoma located in the myometrium and associated with peritoneal inclusion cyst. AJR Am J Roentgenol 2004;182:1531-3. [Crossref] [PubMed]
- Furukawa R, Akahane M, Yamada H, Kiryu S, Sato J, Komatsu S, Inoh S, Yoshioka N, Maeda E, Takazawa Y, Ohtomo K. Endometrial stromal sarcoma located in the myometrium with a low-intensity rim on T2-weighted images: report of three cases and literature review. J Magn Reson Imaging 2010;31:975-9. [Crossref] [PubMed]
- Ozaki K, Gabata T. Magnetic resonance imaging of an endometrial stromal nodule. J Obstet Gynaecol Res 2016;42:99-102. [Crossref] [PubMed]
- Behtash N, Akhavan S, Gilani MM, Mousavi A, Ghaemmaghami F, Mazhari MM. Low grade endometrial stromal sarcoma of uterine: review of 17 cases. Acta Med Iran 2011;49:619-24. [PubMed]
- Geller MA, Argenta P, Bradley W, Dusenbery KE, Brooker D, Downs LS Jr, Judson PL, Carson LF, Boente MP. Treatment and recurrence patterns in endometrial stromal sarcomas and the relation to c-kit expression. Gynecol Oncol 2004;95:632-6. [Crossref] [PubMed]
- Jones KD, Owen E, Berresford A, Sutton C. Endometrial adenocarcinoma arising from endometriosis of the rectosigmoid colon. Gynecol Oncol 2002;86:220-2. [Crossref] [PubMed]
- Zemlyak A, Hwang S, Chalas E, Pameijer CR. Primary extrauterine endometrial stromal cell sarcoma: a case and review. J Gastrointest Cancer 2008;39:104-6. [Crossref] [PubMed]
- Smoot JS, Zaloudek C. Myometrial and stromal lesions of the uterus. Clin Lab Med 1995;15:545-73. [PubMed]
- Kusaka M, Mikuni M, Nishiya M. A case of high-grade endometrial stromal sarcoma arising from endometriosis in the cul-de-sac. Int J Gynecol Cancer 2006;16:895-9. [Crossref] [PubMed]
- La Fianza A, Meloni G, Alberici E, Campani R. Magnetic resonance appearance of endometrial sarcoma: report of a case with unusual findings. Magn Reson Imaging 1999;17:637-40. [PubMed]
- Testa AC, Di Legge A, Bonatti M, Manfredi R, Scambia G. Imaging techniques for evaluation of uterine myomas. Best Pract Res Clin Obstet Gynaecol 2016;34:37-53. [Crossref] [PubMed]
- Ueda M, Otsuka M, Hatakenaka M, Torii Y. Uterine endometrial stromal sarcoma located in uterine myometrium: MRI appearance. Eur Radiol 2000;10:780-2. [Crossref] [PubMed]
- Koyama T, Togashi K, Konishi I, Kobayashi H, Ueda H, Kataoka ML, Kobayashi H, Itoh T, Higuchi T, Fujii S, Konishi J. MR imaging of endometrial stromal sarcoma: correlation with pathologic findings. AJR Am J Roentgenol 1999;173:767-72. [Crossref] [PubMed]
- Wáng YX. Advance modern medicine with clinical case reports. Quant Imaging Med Surg 2014;4:439-43. [PubMed]


