
The relationship of testicular stiffness with Johnsen score and sperm retrieval outcome in men with non-obstructive azoospermia
Introduction
Azoospermia is present in approximately 5–10% of male cases of infertility, with nonobstructive azoospermia (NOA) accounting for 60% of these cases (1,2). Microdissection testicular sperm extraction (micro-TESE) is the preferred technique for testicular sperm retrieval (3), but its success rates for patients with NOA range from 39.1% to 46.8% due to severe spermatogenic dysfunction in the testes (4). As a result, patients with NOA considering micro-TESE typically want to know the likelihood of success beforehand, given the emotional and financial implications of a failed procedure (5). Histopathological examination of testicular tissue using a quantitative histological scoring system, such as the Johnsen score (JS), is commonly used to assess spermatogenesis and predict sperm retrieval rates (SRRs) before micro-TESE. The JS is based on grading multiple tubules from 10 to 1 according to the most predominant histological pattern observed in the biopsy, with a higher score indicating better spermatogenic status and a lower score indicating more severe dysfunction (6).
Although testicular biopsy has been a crucial procedure for many years in diagnosing and managing NOA, the benefits of performing a diagnostic biopsy before micro-TESE are questionable. This is because the diagnostic biopsy itself is an invasive procedure that can lead to many complications, including pain, infection, intratesticular bleeding, scrotal hematoma, intratesticular scar tissue formation, postoperative hypogonadism, and removal of focal areas of spermatogenesis (7). Patients with NOA typically have heterogeneous seminiferous tubules, meaning that the absence of sperm in a single biopsy does not necessarily indicate the absence of sperm in the entire testis (8). Moreover, a diagnostic testicular biopsy can remove focal areas of sperm production, which can ultimately affect the chances of successful sperm retrieval in the future. Considering all these factors, a noninvasive diagnostic method that can help adjust treatment programs, improve surgical success rates, and avoid unnecessary surgical interventions would be of particular value.
Shear wave elastography (SWE) is a widely used noninvasive method for assessing tissue stiffness and is an effective complement to conventional ultrasonography. Testicular elastic modulus, which is associated with sperm concentration, has been found to have a high diagnostic value for evaluating severe spermatogenic dysfunction (9,10). However, there have been few reports on the relationship between testicular stiffness and pathological findings in patients with NOA. Hu et al. (11) conducted a study that divided infertile males into four groups based on the results of testicular biopsies, including normal testicular spermatogenesis, hypospermatogenesis, spermatogenesis arrest, and Sertoli cell-only syndrome. Significant differences in SWE values were observed between these four groups (P<0.01). Similarly, Abdelaal et al. (12) examined patients with NOA undergoing testicular sperm extraction and found that there was a significant difference in SWE values between those with positive sperm retrieval and those with negative sperm retrieval, with a sensitivity of 75.0% and a specificity of 85.7% (P<0.01). However, these studies are limited by their small number of positive cases, and the results may not be generalizable to the wider population. Greater attention and additional research with refined study designs and larger sample sizes should be conducted to confirm these findings and evaluate the clinical utility of SWE in diagnosing and managing NOA.
The purpose of this study was to evaluate the correlation between testicular stiffness and JS and to determine whether SWE can serve as a substitute for testicular biopsy in predicting the efficacy of micro-TESE. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1381/rc).
Methods
Participant screening and enrollment
The prospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Shengjing Hospital of China Medical University (No. 2018PS104J). Informed consent was obtained from all participants. Initially, we recruited 331 consecutive patients with NOA who had visited the reproductive medical center of Shengjing Hospital of China Medical University from January 2018 to October 2021. To minimize selection bias, strict inclusion and exclusion criteria were applied. The inclusion criteria were the following: (I) a diagnosis of NOA according to the updated guidelines (13); (II) completion of testicular SWE examination, with the image exhibiting high clarity and the elastic modulus demonstrating consistent stability; and (III) micro-TESE performed within 7 days after the ultrasound examination, during which a part of testicular tissue was excised for pathological examination.
The exclusion criteria were as follows: (I) a history of testicular trauma; (II) testicular biopsy within the previous 3 months; (III) abnormal ultrasound findings such as testicular masses, extensive microlithiasis, hydrocele, cryptorchidism, and inguinal hernia; (IV) a biopsy sample insufficient in size to evaluate the spermatogenetic status of the testis; (V) a history of mumps orchitis; and (VI) a history of previous chemotherapy or radiotherapy.
Grayscale ultrasound and SWE examinations
Grayscale ultrasound and SWE measures were performed using an Aixplorer ultrasound diagnostic imaging system (SuperSonic Imagine, Aix en Provence, France), with a linear transducer operating at a frequency of 4–15 MHz. All examinations were conducted by an experienced radiographer with 5 years of testicular SWE experience.
Participants were examined in the supine position, and grayscale ultrasound of the scrotal contents was performed to assess the placement, contour, size, and parenchymal echoes of the testes. Testicular length, width, and height were measured, and testicular volume was calculated using the following formula: testicular volume (mL) = (length × width × height) × 0.71 (14).
Following the grayscale ultrasound assessment, SWE measurements were taken in the maximum longitudinal plane. Grayscale and SWE images were displayed simultaneously on the monitor, with the elastic modulus displayed as an SWE map in kilopascals (range, 0–180 kPa). The central part of the testis exhibits a more consistent stiffness, thereby providing a more accurate representation of the overall testicular stiffness (15), and a 10-mm region of interest (ROI) in the middle of testis was selected for three consecutive measurements of the maximum elastic modulus (Emax), mean elastic modulus (Emean), and minimum elastic modulus (Emin) (Figure 1). The average values were recorded as the testicular elastic modulus. If the testicular volume was small, the size of the ROI was adapted accordingly.
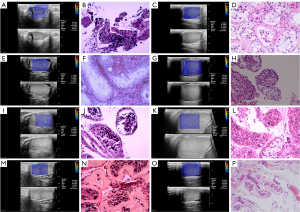
Micro-TESE and testicular pathological analysis
All participants underwent micro-TESE after ultrasound examination, from which partial testicular tissue was removed for biopsy (Figure 1). The testicular samples were then immersed in Bouin’s solution and sent to the pathology laboratory for analysis. The biopsy samples were stained with hematoxylin and eosin and examined under light microscopy. Histopathological analysis of the testicular samples was performed by a single pathologist who was an expert in andrology. The pathologist was blinded to the clinical data and SWE results. Based on the level of spermatogenesis, a JS from 1 to 10 was assigned to each testicular biopsy specimen. Sperm retrieval outcomes were also recorded.
Sample size
The sample size was determined using PASS 15.0 software (Number Cruncher Statistical Software, LLC, Kaysville, UT, USA). Based on previous literature (12) and our preliminary experiment, the area under the receiver operating characteristic (ROC) curves for SWE and JS in distinguishing successful from negative sperm retrieval were found to be 0.869 and 0.750, respectively. We employed tests for two ROC curves to estimate the required sample size. With a power of 90% and an alpha level of 5% (two-sided), the minimum sample size per group was calculated as 65. With a dropout rate of 40%, the total minimum sample size needed was determined to be 182 participants. During our study, we unexpectedly encountered a higher number of patients who did not undergo micro-TESE and testicular biopsy than anticipated; therefore, we included additional patients.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). The normality of the data and the homogeneity of the variables were examined according to the Kolmogorov-Smirnov test. The data did not satisfy the normal distribution and are presented as medians with 25th and 75th percentiles. Statistical differences in parameters between the positive and negative sperm retrieval groups were determined using the Mann-Whitney test. Since JS is a rank variable, Spearman rank correlation analysis was performed to examine the correlations between JS and either testicular volumes or elastic modulus. ROC curves were drawn to evaluate the diagnostic performance of the testicular elastic modulus and testicular volume. The areas under the ROC curves (AUCs), sensitivities, and specificities were calculated, and the cutoff values were determined using the Youden indexes. A P value of <0.05 was considered statistically significant.
Results
Participant screening and enrollment
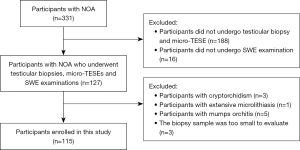
The process of participant screening and enrollment is shown in Figure 2. As it has been reported that the histological features of the testes can vary between the left and right sides (16), we included all pathology results from testicular biopsies in our study. In total, 140 testes from 115 participants were enrolled.
Descriptive statistics
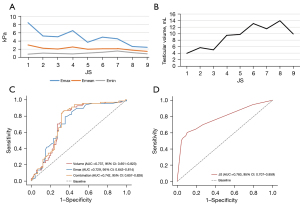
Descriptive statistics of age, testicular volume, elastic modulus, number participants with successful sperm retrieval, autosomal abnormalities, and Y-chromosome azoospermic factor region microdeletions for each participant at different JS levels are presented in Table 1. We observed an increase in testicular volume with increasing JS, while Emax and Emean showed a decrease (Figure 3). No significant changes in Emin or age were observed in our study (Figure 3).
Table 1
| Johnsen score | Patients, N | Testicular volume, mL† | Emax, kPa† | Emean, kPa† | Emin, kPa† | Age, years† | Sperm retrieval success (%, n/N) | Autosomal abnormality and chromosome polymorphic variants | Y chromosome AZF region microdeletions |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 3.92 (1.76, 5.30) |
8.45 (6.43, 14.65) |
3.00 (2.48, 3.90) |
0.75 (0.58, 1.23) |
30.00 (29.25, 31.00) |
22.22 (4/18) | 47,XXY (n=4) | – |
| 2 | 53 | 5.67 (3.37, 7.27) |
5.20 (3.90, 7.00) |
2.20 (1.65, 2.75) |
1.00 (0.80, 1.40) |
30.00 (27.00, 35.50) |
33.96 (18/53) | 47,XXY (n=6) | Microdeletions of sY152, sY254, sY255 |
| 3 | 9 | 5.00 (4.90, 9.29) |
5.00 (3.90, 5.70) |
2.00 (1.80, 2.60) |
0.90 (0.60, 1.45) |
30.00 (28.50, 32.00) |
44.44 (4/9) | 45,XY,der(13;14)(q10;q10) | – |
| 4 | 9 | 9.46 (5.85, 11.35) |
6.50 (4.85, 8.25) |
2.50 (1.50, 4.10) |
0.80 (0.35, 1.65) |
28.00 (26.00, 33.00) |
33.33 (3/9) | 46,XY,(21ps+) (n=2) | – |
| 5 | 4 | 9.74 (6.46, 11.76) |
3.65 (3.23, 4.53) |
1.95 (1.63, 2.28) |
1.00 (0.75, 1.40) |
27.50 (23.25, 28.75) |
75 (3/4) | 46,XY,t(13;14)(p12;q21); 46,XY,t(20;21)(p11.2;q11.2) | – |
| 6 | 4 | 13.09 (7.35, 22.37) |
4.90 (2.33, 9.88) |
2.05 (1.60, 3.33) |
1.20 (0.73, 1.45) |
29.00 (26.00, 32.00) |
50 (2/4) | 46,XY(9qh+) | – |
| 7 | 3 | 11.48 (N/A, N/A)‡ |
4.50 | 2.10 | 1.50 | 27.00 | 66.67 (2/3) | – | Microdeletions of sY152, sY157, sY239, sY242, sY254, sY255 (n=2) |
| 8 | 39 | 13.96 (12.09, 17.35) |
2.60 (2.50, 3.10) |
1.70 (1.50, 1.90) |
1.10 (0.90, 1.50) |
30.00 (27.00, 34.00) |
92.31 (36/39) | 46,XY(1qh+) | – |
| 9 | 1 | 9.89 (N/A, N/A)‡ |
2.40 | 1.4 | 0.8 | 28 | 100 (1/1) | – | – |
| Total | 140 | 7.29 (4.77, 12.09) |
4.55 (2.83, 6.88) |
2.05 (1.60, 2.70) |
1.05 (0.80, 1.40) |
30.00 (27.00, 33.00) |
52.14 (73/140) | – | – |
†, values are presented as the median (25th, 75th percentile); ‡, for groups with fewer participants, only the median is provided due to insufficient data for interquartile range calculation. Emax, maximum elastic modulus; Emean, mean elastic modulus; Emin, minimum elastic modulus; AZF, azoospermic factor; N/A, not applicable.
The association of JS with testicular elastic modulus and testicular volume
The JS was positively correlated with testicular volume but negatively correlated with Emax and Emean, with correlation coefficients of 0.804, −0.686, and −0.456, respectively (P<0.01). The JS was positively correlated with Emin, with the correlation coefficient of 0.182 (P<0.05).
ROC curves of JS, Emax, and testicular volume (Figure 3)
Out of the 140 testes analyzed, for participants with positive sperm retrieval (73 testes, 52.1%), the median (25th, 75th percentile) values of JS, testicular volume, Emax, and Emean were 8 [2, 8], 11.35 (5.53, 14.16) mL, 3.10 (2.55, 5.40) kPa, and 1.90 (1.60, 2.45) kPa, respectively. For participants with negative sperm retrieval (67 testes, 47.9%), the median values of JS, testicular volume, Emax, and Emean were 2 [2, 3], 5.75 (4.32, 7.87) mL, 5.60 (4.00, 7.90) kPa, and 2.20 (1.70, 3.30) kPa, respectively. Significant differences in JS, testicular volume, and Emax were observed between participants with positive and negative sperm retrieval (P<0.01).
We constructed ROC curves to evaluate the diagnostic value of JS, testicular volume, and Emax in distinguishing between participants with positive and negative sperm retrieval. The AUCs were 0.783 [95% confidence interval (CI): 0.707–0.859; P<0.01], 0.737 (95% CI: 0.651–0.823; P<0.01), and 0.729 (95% CI: 0.643–0.814; P<0.01), respectively. The optimal cutoff values were determined using the maximum Youden index. The optimal cutoff value for JS was 4.5, with a sensitivity of 60.3% and a specificity of 89.6%; for Emax, the optimal cutoff value was 3.75 kPa, with a sensitivity of 79.1% and specificity of 64.4%; and for testicular volume, the optimal cutoff value was 8.17 mL, with a sensitivity of 68.5% and specificity of 83.6%. Combining Emax and testicular volume improved the diagnostic value, with an AUC of 0.742 (95% CI: 0.657–0.828; P<0.01) and a sensitivity and specificity of 83.6% and of 68.5%, respectively.
Seminiferous tubule hyalinization
Among the participants with JS 1–2, 20 (20/71) had seminiferous tubule hyalinization, with 10 cases of Klinefelter syndrome (KS) and 10 cases without KS. For those with KS, the median (25th, 75th percentile) values of Emax, Emean, and testicular volume were 9.15 (7.13, 13.43) kPa, 4.60 (3.50, 5.28) kPa, and 1.41 (1.03, 1.49) mL, respectively. For those without KS but with seminiferous tubule hyalinization, the median (25th, 75th percentile) values of Emax, Emean, and testicular volume were 8.75 (6.18, 17.25) kPa, 2.90 (2.10, 3.83) kPa, and 5.38 (4.09, 6.41) mL, respectively.
Mumps orchitis
We excluded five participants with a confirmed history of mumps orchitis due to the extremely high testicular stiffness. The median (25th, 75th percentile) values for these participants were 10.70 (9.15, 21.95) kPa for Emax, 3.60 (2.05, 4.20) kPa for Emean, 7.16 (6.17, 14.51) mL for testicular volume, and 8 [5, 8] for JS. All of these participants had successful sperm retrieval.
Varicocele
In our study, 15 participants were diagnosed with varicocele according to scrotal color Doppler ultrasound. The median (25th, 75th percentile) values for Emax, Emean, testicular volume, and JS were 6.90 (2.40, 13.00) kPa, 2.60 (1.30, 6.70) kPa, 6.48 (1.48, 15.37) mL, and 2 [2, 8], respectively.
Discussion
In recent years, there has been significant interest in developing an effective means to evaluating patients with NOA prior to micro-TESE. Our study discovered a strong correlation between testicular stiffness and pathological findings in patients with NOA. Moreover, we found that combining testicular stiffness with testicular volume led to a highly effective method for identifying patients with NOA who are likely to have positive sperm retrieval during micro-TESE.
Numerous studies have explored the connection between histopathology and SRR in patients with NOA. Histopathology is a potent predictor of the success of micro-TESE in these patients (17). One quantitative histopathological method is the JS, which independently predicts sperm retrieval. Studies have shown that the presence of mature spermatozoa in histopathology specimens, regardless of overall spermatogenesis, strongly predicts successful SRR, with a JS score of ≥8 being the most predictive (5,16). For example, Rohan et al. (18) reported that the mean JS was 7.8 in men with successful micro-TESE compared to 2.84 in those with unsuccessful sperm retrieval. When the optimal JS cutoff value was set to 5, the SRR was 6% for scores <5 and 88% for scores ≥5, which is in line with our results. Although histopathological findings offer a relatively higher accurate prediction of SRR, performing a diagnostic biopsy before micro-TESE is akin to conducting a surgical procedure twice, which patients are reluctant to accept.
Fibrosis, characterized by thickening of the seminiferous tubule wall, is a hallmark of male infertility and a common feature of impaired spermatogenic function (19). The resulting increased stiffness can be detected by SWE, which evaluates the degree of testicular fibrosis and parenchymal damage (20). Many studies have shown that severe oligozoospermia and NOA are associated with increased testicular SWE-assessed stiffness (9,10). In our study, we more deeply examined the relationship between SWE and NOA, discovering that testicular stiffness increased as JS decreased, suggesting a strong correlation between stiffness and the parenchymal damage associated with spermatogenic function. These results suggest that SWE has the potential to replace JS as a predictor of SRR, given the high level of agreement between the two measures.
Testicular volume is a valuable clinical tool for evaluating the severity of male infertility (9), as it has a high predictive value for spermatogenesis, especially in patients with NOA (21). However, testicular volumes in these patients are typically smaller except in cases of early or late maturation arrest, which aligns with our study’s findings (22). Our research revealed a significant positive correlation between testicular volume and JS, with smaller testes being associated with JS scores of 1–3. Although testicular volume has been linked to the success rate of micro-TESE in some studies (23), others have reached conflicting conclusions. For instance, one study found that SRRs did not differ significantly among men with testicular volumes of ≤2, >2–10, and >10 mL (24). Moreover, a recent meta-analysis of 1,764 cases found no threshold of testicular volume associated with SRR (25). Additionally, testicular volume varies widely due to numerous factors, including race (26). Although testicular volume is a useful diagnostic tool for evaluating spermatogenic function in patients with NOA, its role as an independent predictor of successful sperm retrieval remains controversial.
Our study found that combining Emax and testicular volume was similarly effective to using JS in predicting successful sperm retrieval in participants with NOA. Our results suggest that testicular elastic modulus combined with volume could serve as a noninvasive alternative to testicular biopsy for predicting SRR before micro-TESE.
The etiology of NOA is a crucial factor in predicting successful sperm retrieval. Our study found that quantitative SWE features demonstrated good diagnostic performance in NOA patients with mumps orchitis and KS. Additionally, we found that patients with NOA caused by mumps orchitis exhibited characteristic SWE features of ultrahigh testicular stiffness, with median Emax and Emean values of 10.70 and 3.60 kPa, respectively. These values were considerably higher than those observed in the NOA participants enrolled in our study, who had median Emax and Emean values of 4.55 and 2.05 kPa, respectively. These findings are consistent with previous reports indicating that patients with orchitis have the highest and most effective SRR of 100%, while that of other patients is an average 46.0% (27). Our study also showed that the median JS of patients with NOA and mumps orchitis was 8, and the SRR was 100%. However, due to the limited number of cases in this study, further research with a larger sample size is necessary to validate these findings.
KS (karyotype 47, XXY) remains the most common chromosomal disorder that causes NOA. The testes of individuals with KS are characterized by extensive fibrosis and hyalinization of the seminiferous tubules, as well as hyperplasia of the interstitium (28). The increased stiffness of KS testes is due to seminiferous tubule hyalinization. However, in our study, we observed obvious seminiferous tubule hyalinization in the testes of 10 participants without KS who had a JS of 1–2, which is consistent with previous research (29). Seminiferous tubule hyalinization has been reported not only in the testes of those with KS, but also in the undescended testes and testes of patients with severe oligozoospermia or NOA (30). Moreover, in our study, participants without KS but with significant seminiferous tubule hyalinization had testicular stiffness similar to that of KS participants but larger testicular volumes. Therefore, a combination of testicular stiffness and volume showed high diagnostic efficacy in distinguishing KS from other causes of NOA.
We did not exclude participants with varicocele from the analysis for several reasons. First, among participants with the same JS, we observed no differences in testicular volume or elastic modulus between those with varicocele and those without. This suggests that varicocele did not significantly affect testicular pathology in our study population. Second, to minimize the potential impact of varicocele on testicular biopsy results, we only performed biopsies on the contralateral testes. Therefore, we believe that varicocele did not have a significant effect on our study findings.
Our study had several limitations that need to be considered. First, even though participants with NOA had significant impairments in their sperm production, it is important to note that there could be regions within the testicular tissue that still exhibit active spermatogenesis. Therefore, a single testicular biopsy may not accurately reflect the overall spermatogenic function of the entire testis. Second, since all the ultrasound and SWE examinations were performed by a single operator, we could not assess interobserver variability.
Conclusions
Our study suggests that the combination of testicular stiffness and volume measurements may serve as a viable alternative to the pathological JS in predicting the likelihood of successful sperm retrieval prior to micro-TESE procedures.
Acknowledgments
We acknowledge and thank the triage nurses for assisting us with the data collection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1381/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1381/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Shengjing Hospital of China Medical University (No. 2018PS104J). Written informed consent was provided by all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm. Management of nonobstructive azoospermia: a committee opinion. Fertil Steril 2018;110:1239-45. [Crossref] [PubMed]
- Wosnitzer M, Goldstein M, Hardy MP. Review of Azoospermia. Spermatogenesis 2014;4:e28218. [Crossref] [PubMed]
- Flannigan R, Bach PV, Schlegel PN. Microdissection testicular sperm extraction. Transl Androl Urol 2017;6:745-52. [Crossref] [PubMed]
- Achermann APP, Pereira TA, Esteves SC. Microdissection testicular sperm extraction (micro-TESE) in men with infertility due to nonobstructive azoospermia: summary of current literature. Int Urol Nephrol 2021;53:2193-210. [Crossref] [PubMed]
- Abdel Raheem A, Garaffa G, Rushwan N, De Luca F, Zacharakis E, Abdel Raheem T, Freeman A, Serhal P, Harper JC, Ralph D. Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia. BJU Int 2013;111:492-9. [Crossref] [PubMed]
- Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1970;1:2-25. [Crossref] [PubMed]
- Eliveld J, van Wely M, Meißner A, Repping S, van der Veen F, van Pelt AMM. The risk of TESE-induced hypogonadism: a systematic review and meta-analysis. Hum Reprod Update 2018;24:442-54. [Crossref] [PubMed]
- Cito G, Coccia ME, Dabizzi S, Morselli S, Della Camera PA, Cocci A, Criscuoli L, Picone R, De Carlo C, Nesi G, Micelli E, Serni S, Carini M, Natali A. Relevance of testicular histopathology on prediction of sperm retrieval rates in case of non-obstructive and obstructive azoospermia. Urologia 2018;85:60-7. [Crossref] [PubMed]
- Cui J, Du Q, Fu W. Application of real-time shear wave elastography in the assessment of male infertility. Quant Imaging Med Surg 2022;12:1505-16. [Crossref] [PubMed]
- Erdoğan H, Durmaz MS, Özbakır B, Cebeci H, Özkan D, Gökmen İE. Experience of using shear wave elastography in evaluation of testicular stiffness in cases of male infertility. J Ultrasound 2020;23:529-34. [Crossref] [PubMed]
- Hu JY, Huang WL, Gao Y, Yang Z, Ding L, Xie Y, Xie XY, Hu HT, Wang Z. Preliminary investigation of the diagnostic value of shear wave elastography in evaluating the testicular spermatogenic function in patients with azoospermia. Andrologia 2021;53:e14039. [Crossref] [PubMed]
- Abdelaal AMA, El-Azizi HM. GamalEl Din SF, Abdulsalam Mohammad Azzazi O, Shokr Mohamed M. Evaluation of the potential role of shear wave elastography as a promising predictor of sperm retrieval in non-obstructive azoospermic patients: A prospective study. Andrology 2021;9:1481-9. [Crossref] [PubMed]
- Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, Mulhall JP, Niederberger C, Sandlow JI, Sokol RZ, Spandorfer SD, Tanrikut C, Treadwell JR, Oristaglio JT, Zini A. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. J Urol 2021;205:36-43. [Crossref] [PubMed]
- Freeman S, Bertolotto M, Richenberg J, Belfield J, Dogra V, Huang DY, et al. Ultrasound evaluation of varicoceles: guidelines and recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for detection, classification, and grading. Eur Radiol 2020;30:11-25. [Crossref] [PubMed]
- Fang C, Huang DY, Sidhu PS. Elastography of focal testicular lesions: current concepts and utility. Ultrasonography 2019;38:302-10. [Crossref] [PubMed]
- Tang WH, Zhou SJ, Song SD, He HY, Wu H, Zhang Z, Yang YZ, Zhang HL, Mao JM, Liu DF, Zhao LM, Lin HC, Hong K, Ma LL, Zhuang XJ, Jiang H. A clinical trial on the consistency of bilateral testicular tissue histopathology and Johnsen score: single side or bilateral side biopsy? Oncotarget 2018;9:23848-59. [Crossref] [PubMed]
- Toksoz S, Kizilkan Y. Comparison of the Histopathological Findings of Testis Tissues of Non-Obstructive Azoospermia with the Findings after Microscopic Testicular Sperm Extraction. Urol J 2019;16:212-5. [Crossref] [PubMed]
- Rohan P, Daly N, O'Kelly A, O'Leary M, Dineen T, Shah N, Daly P, Waterstone J, Cullen I. Evaluation of Microdissection Testicular Sperm Extraction (mTESE), Outcomes and Predictive Factors in Ireland: The Gold Standard for Men with Non-Obstructive Azoospermia. J Reprod Infertil 2021;22:103-9. [Crossref] [PubMed]
- Shiraishi K, Takihara H, Naito K. Quantitative analysis of testicular interstitial fibrosis after vasectomy in humans. Aktuelle Urol 2003;34:262-4. [Crossref] [PubMed]
- Fuschi A, Capone L, Abuorouq S, Al Salhi Y, Velotti G, Aversa S, Carbone F, Maceroni P, Petrozza V, Carbone A, Pastore AL, Porta N. Shear wave elastography in varicocele patients: Prospective study to investigate correlation with semen parameters and histological findings. Int J Clin Pract 2021;75:e13699. [Crossref] [PubMed]
- Mancini M, Carrafiello G, Melchiorre F, Pelliccione F, Andreassi A, Mantellassi G, Ahmed Said Z, Pecori Giraldi F, Banderali G, Folli F. Early varicocelectomy by percutaneous scleroembolization improves seminiferous tubules spermatozoa release in the adolescent phase of testicular growth. Andrologia 2019;51:e13286. [Crossref] [PubMed]
- Tsai MC, Cheng YS, Lin TY, Yang WH, Lin YM. Clinical characteristics and reproductive outcomes in infertile men with testicular early and late maturation arrest. Urology 2012;80:826-32. [Crossref] [PubMed]
- Kizilkan Y, Toksoz S, Turunc T, Ozkardes H. Parameters predicting sperm retrieval rates during microscopic testicular sperm extraction in nonobstructive azoospermia. Andrologia 2019;51:e13441. [Crossref] [PubMed]
- Bryson CF, Ramasamy R, Sheehan M, Palermo GD, Rosenwaks Z, Schlegel PN. Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction. J Urol 2014;191:175-8. [Crossref] [PubMed]
- Li H, Chen LP, Yang J, Li MC, Chen RB, Lan RZ, Wang SG, Liu JH, Wang T. Predictive value of FSH, testicular volume, and histopathological findings for the sperm retrieval rate of microdissection TESE in nonobstructive azoospermia: a meta-analysis. Asian J Androl 2018;20:30-6. [Crossref] [PubMed]
- Takihara H, Sakatoku J, Fujii M, Nasu T, Cosentino MJ, Cockett AT. Significance of testicular size measurement in andrology. I. A new orchiometer and its clinical application. Fertil Steril 1983;39:836-40. [Crossref] [PubMed]
- Zhang HL, Zhao LM, Mao JM, Liu DF, Tang WH, Lin HC, Zhang L, Lian Y, Hong K, Jiang H. Sperm retrieval rates and clinical outcomes for patients with different causes of azoospermia who undergo microdissection testicular sperm extraction-intracytoplasmic sperm injection. Asian J Androl 2021;23:59-63. [Crossref] [PubMed]
- Klinefelter HF, Reifenstein EC, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without A-Leydigism, and increased excretion of follicle-stimulating hormone. J Clin Endocrinol 1942;2:615-27.
- Spahovic H, Alic J, Göktolga Ü, Lepara Z, Lepara O, Rama A, Suljevic I. "Second-look" Micro Testicular Sperm Extraction (MicroTESE) in Patients with Non-obstructive Azoospermia Following Histopathological Analysis. Med Arch 2020;74:279-84. [Crossref] [PubMed]
- Haider SG, Talati J, Servos G. Ultrastructure of peritubular tissue in association with tubular hyalinization in human testis. Tissue Cell 1999;31:90-8. [Crossref] [PubMed]


