
Semiquantitative assessment of preganglionic nerves for chronic immune-mediated neuropathies using brachial plexus magnetic resonance imaging
Introduction
Chronic immune-mediated peripheral neuropathies are disorders caused by autoimmune responses against peripheral nerves. Chronic inflammatory demyelinating polyneuropathy (CIDP) and multifocal motor neuropathy (MMN) are representative subtypes (1,2). According to the latest update of the European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) CIDP diagnostic guideline, patients with anti-neurofascin-155 (NF155)-positive autoimmune nodopathy (AN) (NF155+) have been identified as a distinct cohort independent of CIDP due to characteristic pathologic changes and clinical manifestations (3). Because treatment methods differ, it is critical to diagnose and differentiate these various subtypes. The diagnosis depends on typical clinical manifestations, specific nerve conduction studies (NCSs), and laboratory features (3-5). However, assessment of NCS in the proximal areas of the brachial plexus may not always be accurate. Furthermore, laboratory examinations such as cerebrospinal fluid (CSF) analysis and nerve biopsy can cause trauma.
Brachial plexus magnetic resonance imaging (MRI) can be used to provide supporting diagnostic criteria, especially for those without evident NCS abnormalities (3,5). In studies of postganglionic nerves, features such as nerve root hypertrophy, increased signal intensity on the T2-weighted (T2W) sequence, and nerve tissue function detected on diffusion tensor imaging (DTI) have been found to be important supplementary evidence for identifying variant subtypes (6-9). The preganglionic part of the brachial plexus nerve is critical to the functional integrity of the upper extremity. Compared to the postganglionic nerves, the preganglionic nerves are delicate and immersed in CSF. However, to the best of our knowledge, few studies have focused on preganglionic nerves and no assessment standards for preganglionic nerve thickening. Therefore, the aim of our study was to develop a feasible and repeatable semiquantitative assessment of enlargement of the brachial plexus preganglionic nerves and to investigate the differences in preganglionic nerve root thickening among patients with NF155+, CIDP, and MMN and healthy controls. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1473/rc).
Methods
Recruitment of patients and controls
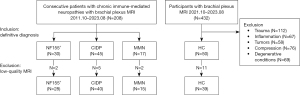
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of Huashan Hospital of Fudan University (No. 2022-140). Informed consent was obtained from each patient. We retrospectively collected the data of consecutive patients who were diagnosed with NF155+, CIDP, or MMN and who underwent brachial plexus MR examination at Huashan Hospital Fudan University between October 2011 and August 2023. CIDP and MMN were diagnosed according to the European Federation of Neurological Societies (EFNS)/PNS and EAN/PNS criteria (3,5). As described in a previous study, the diagnosis of NF155+ was clinically and serologically confirmed. Anti-NF155 antibodies in the serum samples were detected with cell-based assays (CBAs) and confirmed by immunofluorescence tissue-based assays (TBAs) (10). We reviewed all participants who underwent brachial plexus MRI during this period and excluded those with a history of trauma, inflammation, tumors, compression, or degenerative conditions. The remaining participants, without a history of additional conditions, were included as healthy controls. The exclusion criteria were inadequate image quality caused by motion, CSF flow, and significant cervical disc herniation. Figure 1 shows the flowchart of the study.
MRI protocol
MRI was performed with a 3.0 T Discovery MR750 (GE HealthCare, Milwaukee, WI, USA) or a 3.0 T MAGNETOM Verio device (Siemens Healthineers, Erlangen, Germany) and use of a neck and body coil. The technical parameters for the MRI were as follows: (I) a three-dimensional (3D) fast imaging employing steady-state acquisition (FIESTA), with repetition time/echo time =4.3/1.7 ms, pixel bandwidth =244 kHz, slice thickness =1.0 mm, field of view =26×26 cm2, matrix =260×260, and echo chain length =1; and (II) coronal T2W sampling perfection with application-optimized contrast using different flip angle evolutions (SPACE) sequence, with repetition time/echo time =1,500/223 ms, pixel bandwidth =488 kHz, slice thickness =2.0 mm, field of view =18×18 cm2, matrix =320×309, and echo chain length =107.
MRI analysis
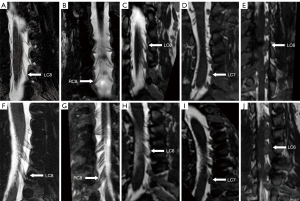
In this study, the C5–C8 preganglionic nerves were analyzed. T1 nerve was not included in the analysis because it is vulnerable to gas artifacts of the lung apex. Two experienced radiologists, both with more than 10 years of experience in neuromuscular imaging, underwent 3 months of specialized training for the newly proposed semiquantitative assessment. They were blinded to the clinical data of patients, reviewed 3D-FIESTA and T2W-SPACE images, and evaluated the grade of preganglionic thickening. The degree of preganglionic enlargement was defined semiquantitatively as follows: 0 points, normal; 1 point, fascicular thickening; 2 points, adhesive thickening less than half of the length of the longitudinal axis; 3 points, adhesive thickening more than half and less than the full length of the longitudinal axis; and 4 points, adhesive thickening in the full length of the longitudinal axis (Figure 2). Both ventral and dorsal nerve roots were assessed. Any discrepancies between the two radiologists were resolved by discussion. The sum score of both sides ranged from 0 to 32, and definite enlargement was defined as a sum score ≥20.
Statistical analysis
We used the Shapiro-Wilk test to assess the normality of continuous variables. Continuous variables are presented as the median with the interquartile range and categorical variables as numbers with percentages. The Kruskal-Wallis test was used to ascertain the differences between multiple groups of independent continuous variables or ordinal categorical variables. Pearson χ2 test or Fisher exact test was used to analyze dichotomous variables. A two-sided P value <0.05 was considered significant. For multiple comparisons (six pairs for four groups), the significance level was defined as a P value of 0.008 after Bonferroni correction. Given that the grade of preganglionic thickening was an ordinal categorical variables, the kappa (κ) coefficient was used to assess inter- and intraobserver agreement. Statistical analyses of inter- and intraobserver agreements were interpreted by the Landis and Koch interpretation as follows: fair, 0.21–0.40; moderate, 0.41–0.60; substantial, 0.61–0.80; and almost perfect, ≥0.81 (11). All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Demographic characteristics of patients and controls
In this study, a total of 83 patients and 39 healthy controls were included. Of these, 28 patients had NF155+, 40 had CIDP, and 15 had MMN. The sample consisted of 84 men and 38 women, with a median age of 41 years and an age range of 11 to 78 years. The demographic features are summarized in Table 1. After the Shapiro-Wilk test, we found that the ages of those with NF155+ did not follow a normal distribution. Moreover, these patients were younger than those with CIDP or MMN patients and healthy controls (Kruskal-Wallis test; P<0.001, P=0.008, and P<0.001, respectively), while there was no significant difference in the mean age between the other three groups. In addition, the proportion of males in patients with NF155+ was higher than that in those with CIDP (89% vs. 58%; χ2 test; P=0.02), whereas there was no significant difference in the proportion of males and females in the other groups.
Table 1
| Demographic characteristics | NF155+ (n=28) | CIDP (n=40) | MMN (n=15) | HC (n=39) | P value |
|---|---|---|---|---|---|
| Age (years) | 22.5 [16–36] | 46 [26.5–54] | 39 [34–57] | 46 [31–55] | <0.001 |
| Sex | 0.02 | ||||
| Female | 3 [11] | 17 [43] | 3 [20] | 15 [38] | |
| Male | 25 [89] | 23 [58] | 12 [80] | 24 [62] |
Values are presented as the median [interquartile range] or number [%]. NF155+, anti-neurofascin-155-antibody-positive autoimmune nodopathy; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; MMN, multifocal motor neuropathy; HC, healthy control.
Comparison of bilateral C5–C8 preganglionic single-nerve scores among groups
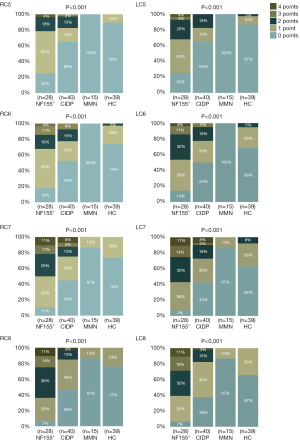
Based on the above semiquantitative assessment of the preganglionic nerves, we assessed the bilateral C5–C8 in each sample individually. First, there was a significant difference in single-nerve score distribution among the four groups (χ2 test; P<0.001). Second, the bilateral C5–C8 scores of the NF155+, CIDP, and MMN groups were significantly different from each other, representing a gradated decrease in a single-nerve score from NF155+ to CIDP to MMN. Finally, there was no significant difference between patients with MMN and healthy controls in the bilateral C5–C8 and also no significant difference in the scores between patients with CIDP and healthy controls except for in the RC7 and RC8 (Figure 3, Table S1).
Comparison of bilateral C5–C8 preganglionic nerve sum scores among the groups
The median of the sum scores was 11 in patients with NF155+ (range, 0–32), 4 in those with CIDP (range, 0–22), 0 in those with MMN (range, 0–4), and 2 in healthy controls (range, 0–11). The NF155+ group had higher sum scores than did the CIDP, MMN, and healthy control groups (Kruskal-Wallis test, P=0.003, P<0.001, and P<0.001, respectively). In addition, the sum score in patients with CIDP was higher than that in patients with MMN (Kruskal-Wallis test; P=0.005). Six patients with NF155+ (21%) and two of those with CIDP (5%) were considered to have definite enlargement (Figure 4). The proportion of those with definite enlargement of preganglionic nerves was higher in the NF155+ group than in the healthy controls (21% vs. 0%, χ2 test, P=0.004), and the proportion of sum score at 0 points was lower in NF155+ patients than CIDP, MMN, and healthy controls (7% vs. 37%, 87%, and 41%; χ2 test; P<0.001) (Table 2). Furthermore, we found that of the 488 pairs of preganglionic nerves in the 122 samples, 441 pairs (90%) had the same left- and right-side scores.
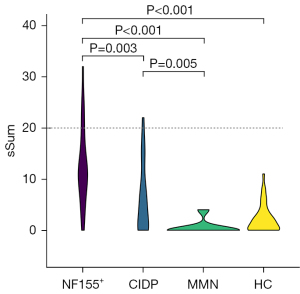
Table 2
| Extent of enlargement | NF155+ (n=28) | CIDP (n=40) | MMN (n=15) | HC (n=39) | P value |
|---|---|---|---|---|---|
| Enlargement | 0.004 | ||||
| Nondefinite | 22 [79] | 38 [95] | 15 [100] | 39 [100] | |
| Definite | 6 [21] | 2 [5] | 0 [0] | 0 [0] | |
| sSum | <0.001 | ||||
| 0 points | 2 [7] | 15 [37] | 13 [87] | 16 [41] | |
| >0 points | 26 [93] | 25 [63] | 2 [13] | 23 [59] |
Values are presented as number [%]. Definite enlargement was defined as a sSum ≥20. NF155+, anti-neurofascin-155-antibody-positive autoimmune nodopathy; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; MMN, multifocal motor neuropathy; HC, healthy control; sSum, sum score.
Inter- and intraobserver agreement in the semiquantitative assessment of preganglionic nerve enlargement
For intraobserver agreement, the κ values for the eight preganglionic nerves (bilateral C5–C8) ranged from 0.68 to 0.76, indicating a substantial agreement among repeated measurements by the same observer. In terms of interobserver agreement, the κ values were lower, ranging from 0.53 to 0.64, which indicated moderate to substantial agreement between different observers (Table 3).
Table 3
| Assessment | κ value | 95% CI |
|---|---|---|
| Intraobserver | ||
| RC5 | 0.69 | 0.57–0.81 |
| RC6 | 0.72 | 0.61–0.82 |
| RC7 | 0.68 | 0.57–0.78 |
| RC8 | 0.76 | 0.66–0.85 |
| LC5 | 0.71 | 0.60–0.81 |
| LC6 | 0.69 | 0.58–0.79 |
| LC7 | 0.72 | 0.62–0.82 |
| LC8 | 0.73 | 0.64–0.83 |
| Interobserver | ||
| RC5 | 0.57 | 0.44–0.70 |
| RC6 | 0.61 | 0.48–0.73 |
| RC7 | 0.53 | 0.41–0.65 |
| RC8 | 0.59 | 0.48–0.71 |
| LC5 | 0.59 | 0.46–0.71 |
| LC6 | 0.64 | 0.53–0.76 |
| LC7 | 0.60 | 0.48–0.71 |
| LC8 | 0.55 | 0.43–0.67 |
κ, kappa; CI, confidence interval; R, right; L, left.
Discussion
In this study, we evaluated a novel semiquantitative assessment for brachial plexus preganglionic nerve enlargement. We observed a pronounced enlargement of the preganglionic nerve in patients with NF155+, and a descending order of sum scores was apparent in patients with NF155+, CIDP, and MMN. Whereas, no significant differences in single-nerve scores or sum scores were observed between patients with MMN patients and healthy controls. In addition, the proportion of definite enlargement was higher in patients with NF155+ than in healthy controls, and the proportion of those with a sum score of 0 was lower in those with NF155+ than in the other three groups.
MRI techniques, such as FIESTA, SPACE, and volumetric isotropic turbo spin echo acquisition (VISTA) sequences, can provide a dark signal for nerve root contrast via a significantly high signal for CSF and can enable high-resolution isotropic 3D acquisitions for multiplanar reconstruction (12). These sequences facilitate the systematic evaluation of preganglionic nerve morphology. Prior to our study, there were a limited number of studies on the MRI characterization of the preganglionic nerves in immune-mediated neuropathies. A few studies (13-15) highlighted the thickening of the preganglionic nerve in patients with Guillain-Barré syndrome (GBS). In addition, a recent study investigated the characterization of preganglionic nerves in chronic inflammatory neuropathies by measuring the diameter of nerves in the transversal plane (16). Given that such intricate anatomical structures require quantitative measurements in which the impact of human factors cannot be overlooked, we developed a semiquantitative method that is both reproducible and reliable.
Earlier studies focusing on postganglionic nerve root demonstrated that patients with NF155+ have significantly greater symmetrical enlargement in the postganglionic nerve root compared to patients with CIDP (17,18). Moreover, the extent of nerve swelling is greater in CIDP than in MMN (6), and the postganglionic nerves of those with MMN patients tend to show asymmetric enlargement (19). Nerve enlargement can be attributed to nerve root hypertrophy and Schwann cell proliferation, which result from repeated demyelination and remyelination (20). Histologic examination of biopsied sural nerves in patients with NF155+, in contrast to those with CIDP, show subperineurial edema and occasional paranodal demyelination without vasculitis, inflammatory cell infiltration, or onion bulbs. Loss of myelin fibers is minimal, even years after the onset of the disease (21,22).
Few studies have performed quantitative analyses of the preganglionic nerves. A study by van Rosmalen et al. (16) reported the thickening of preganglionic nerves in chronic immune neuropathies, particularly noting that the ventral nerve roots in patients with MMN and CIDP with a motor phenotype were thicker than those in CIDP with a sensory-motor phenotype. However, our study observed almost no thickening in the preganglionic nerves of those with MMN. This discrepancy might be attributed to a few factors. The relatively small sample size of MMN cases in our study compared to that of Jongbloed et al. (15 vs. 27) and the uneven distribution of MMN lesions also need to be considered (19). Unlike postganglionic nerves, the preganglionic nerves are immersed in the CSF and are affected by its proteins and inflammatory factors. Notably, there are significant differences in CSF protein levels among these three diseases, with these levels being markedly higher in patients with NF155+ than in those with CIDP and MMN tending to be in the normal range (1,17,21). The correlation between CSF proteins and preganglionic nerve enlargement needs to be investigated in further studies. Besides extremely high levels of CSF protein, upregulation of both T helper type 1 (Th1) and Th2 cytokines is characteristic of NF155+, which causes inflammation at the spinal roots, resulting in nerve root hypertrophy (23). In this study, although not differences were statistically significant difference, there was a tendency that definite enlargement was more likely to be present in the NF155+ group than in the other groups. Thus, the measurement of anti-NF155 antibodies is recommended in those with definite enlargement. In addition, a few nerve roots in healthy controls were scored as 1 or 2 points, possibly due to minor edema of the preganglionic nerves induced by the herniated disc compression.
There are several limitations to this study which should be mentioned. First, the sample size of the study was relatively small, and the demographic and disease conditions of the samples were not harmonized. Second, the assessment of the preganglionic nerve combined the motor ventral roots and sensory dorsal roots. Finally, the effectiveness of semiquantitative assessment is limited by the subjective nature of the assessment and requires specialized professional training. In future research, we will expand the sample size and improve the study methodology, including evaluating ventral and dorsal roots separately to improve the robustness and precision of the findings in characterizing peripheral neuropathy.
Conclusions
Our novel semiquantitative assessment of the brachial plexus preganglionic nerve may be useful in brachial plexus MRI analysis. The single-nerve scores and sum scores decreased, respectively, in patients with NF155+, CIDP, and MMN, but there was no significant difference between patients with MMN and healthy controls. When a brachial plexus MRI reveals a definite enlargement of the preganglionic nerve, NF155+ should be considered in the clinical diagnosis.
Acknowledgments
Funding: This study received funding from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1473/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1473/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Huashan Hospital of Fudan University (No. 2022-140). Informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kieseier BC, Mathey EK, Sommer C, Hartung HP. Immune-mediated neuropathies. Nat Rev Dis Primers 2018;4:31. [Crossref] [PubMed]
- Briani C, Cocito D, Campagnolo M, Doneddu PE, Nobile-Orazio E. Update on therapy of chronic immune-mediated neuropathies. Neurol Sci 2022;43:605-14. [Crossref] [PubMed]
- Van den Bergh PYK, van Doorn PA, Hadden RDM, Avau B, Vankrunkelsven P, Allen JA, Attarian S, Blomkwist-Markens PH, Cornblath DR, Eftimov F, Goedee HS, Harbo T, Kuwabara S, Lewis RA, Lunn MP, Nobile-Orazio E, Querol L, Rajabally YA, Sommer C, Topaloglu HA. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. J Peripher Nerv Syst 2021;26:242-68. [Crossref] [PubMed]
- Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry 2019;90:981-7. [Crossref] [PubMed]
- European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society--first revision. J Peripher Nerv Syst 2010;15:295-301. [Crossref] [PubMed]
- Oudeman J, Eftimov F, Strijkers GJ, Schneiders JJ, Roosendaal SD, Engbersen MP, Froeling M, Goedee HS, van Doorn PA, Caan MWA, van Schaik IN, Maas M, Nederveen AJ, de Visser M, Verhamme C. Diagnostic accuracy of MRI and ultrasound in chronic immune-mediated neuropathies. Neurology 2020;94:e62-74. [Crossref] [PubMed]
- van Rosmalen MHJ, Goedee HS, van der Gijp A, Witkamp TD, van Eijk RPA, Asselman FL, van den Berg LH, Mandija S, Froeling M, Hendrikse J, van der Pol WL. Quantitative assessment of brachial plexus MRI for the diagnosis of chronic inflammatory neuropathies. J Neurol 2021;268:978-88. [Crossref] [PubMed]
- Su X, Kong X, Kong X, Zhu Q, Lu Z, Zheng C. Multisequence magnetic resonance neurography of brachial and lumbosacral plexus in chronic inflammatory demyelinating polyneuropathy: correlations with electrophysiological parameters and clinical features. Ther Adv Neurol Disord 2023;16:17562864221150540. [Crossref] [PubMed]
- Wang S, Man X, Chen Y, Gong T, Gao F, Chen W, Wang G, Zhao B, Chhabra A. Three-dimensional magnetic resonance neurography aids in detection of brachial plexus nerve root signal and size alterations in patients with amyotrophic lateral sclerosis: a case-control study. Quant Imaging Med Surg 2023;13:8694-703. [Crossref] [PubMed]
- Liu B, Zhou L, Sun C, Wang L, Zheng Y, Hu B, Qiao K, Zhao C, Lu J, Lin J. Clinical profile of autoimmune nodopathy with anti-neurofascin 186 antibody. Ann Clin Transl Neurol 2023;10:944-52. [Crossref] [PubMed]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74.
- Widmann G, Henninger B, Kremser C, Jaschke W. MRI Sequences in Head & Neck Radiology - State of the Art. Rofo 2017;189:413-22. [Crossref] [PubMed]
- Berciano J, Sedano MJ, Pelayo-Negro AL, García A, Orizaola P, Gallardo E, Lafarga M, Berciano MT, Jacobs BC. Proximal nerve lesions in early Guillain-Barré syndrome: implications for pathogenesis and disease classification. J Neurol 2017;264:221-36. [Crossref] [PubMed]
- Yikilmaz A, Doganay S, Gumus H, Per H, Kumandas S, Coskun A. Magnetic resonance imaging of childhood Guillain-Barre syndrome. Childs Nerv Syst 2010;26:1103-8. [Crossref] [PubMed]
- Mulkey SB, Glasier CM, El-Nabbout B, Walters WD, Ionita C, McCarthy MH, Sharp GB, Shbarou RM. Nerve root enhancement on spinal MRI in pediatric Guillain-Barré syndrome. Pediatr Neurol 2010;43:263-9. [Crossref] [PubMed]
- van Rosmalen MHJ, Froeling M, Mandija S, Hendrikse J, van der Pol WL, Stephan Goedee H. MRI of the intraspinal nerve roots in patients with chronic inflammatory neuropathies: abnormalities correlate with clinical phenotypes. J Neurol 2022;269:3159-66. [Crossref] [PubMed]
- Wang W, Liu C, Li W, Zhang D, Shan Y, Zheng J, Shan J, Zhao Y, Yan C, Wang Q. Clinical and diagnostic features of anti-neurofascin-155 antibody-positive neuropathy in Han Chinese. Ann Clin Transl Neurol 2022;9:695-706. [Crossref] [PubMed]
- Ogata H, Yamasaki R, Hiwatashi A, Oka N, Kawamura N, Matsuse D, Kuwahara M, Suzuki H, Kusunoki S, Fujimoto Y, Ikezoe K, Kishida H, Tanaka F, Matsushita T, Murai H, Kira J. Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy. Ann Clin Transl Neurol 2015;2:960-71. [Crossref] [PubMed]
- Jongbloed BA, Bos JW, Rutgers D, van der Pol WL, van den Berg LH. Brachial plexus magnetic resonance imaging differentiates between inflammatory neuropathies and does not predict disease course. Brain Behav 2017;7:e00632. [Crossref] [PubMed]
- Bradley LJ, Wilhelm T, King RH, Ginsberg L, Orrell RW. Brachial plexus hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy. Neuromuscul Disord 2006;16:126-31. [Crossref] [PubMed]
- Wang L, Pan J, Meng H, Yang Z, Zeng L, Liu J. Anti-NF155/NF186 IgG4 Antibody Positive Autoimmune Nodopathy. Brain Sci 2022;12:1587. [Crossref] [PubMed]
- Kira JI, Yamasaki R, Ogata H. Anti-neurofascin autoantibody and demyelination. Neurochem Int 2019;130:104360. [Crossref] [PubMed]
- Kira JI. Anti-Neurofascin 155 Antibody-Positive Chronic Inflammatory Demyelinating Polyneuropathy/Combined Central and Peripheral Demyelination: Strategies for Diagnosis and Treatment Based on the Disease Mechanism. Front Neurol 2021;12:665136. [Crossref] [PubMed]


