Clinical application of the retrograde track method in reopening dysfunctional biliary stents
Introduction
Malignant biliary obstruction (MBO) is often caused by various types of tumors, such as cholangiocarcinoma, pancreatic head carcinoma, gallbladder carcinoma, ampullary carcinoma, and metastatic lymph nodes (1). Noncovered metal biliary stent placement (BSP) is a widely recognized and favored drainage method for the treatment of unresectable malignant biliary stenosis (2,3). However, BSP can only mechanically relieve high bile duct pressure and has no therapeutic effect on the primary tumors; moreover, over time, factors such as tumor in-growth, bile duct sludge formation (food residue or unknown origin), and biofilm formation often result in a median stent patency time of only approximately 3–6 months (4,5). Therefore, the long-term efficacy of treatments for malignant biliary stenosis urgently needs to be improved. When biliary duct obstruction reoccurs, patients may experience clinical symptoms such as obstructive jaundice, fever, and pain. It remains unclear if dysfunctional biliary stent can be resolved by reopening of the dysfunctional occluded duct and completing advanced antitumor treatment, such as biliary radiofrequency ablation (6), photodynamic therapy (7), iodione-125 (125I) seed strand brachytherapy (8), or reimplantation of a second stent (9). The first step is to pass the catheter guide wire through the dysfunctional biliary stent in anterograde fashion and reopen the lumen, but the bile duct above the obstruction may be severely dilated, thus making it too difficult for the catheter and guide wire to be manipulated into the dysfunctional stent through the port of the stent under two-dimensional fluoroscopy. A new retrograde track method (RTM) has been used at our center, the First Affiliated Hospital of Zhengzhou University, to reopen dysfunctional biliary stents with 100% technical success, and a retrospective study was conducted to examine the efficacy of this new method. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1030/rc).
Methods
Patients
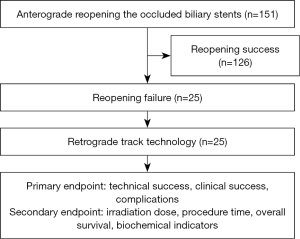
From February 2013 to January 2020, 151 patients underwent percutaneous transhepatic biliary interventional procedures to reopen dysfunctional biliary stents at the First Affiliated Hospital of Zhengzhou University, and 25 patients (12 females, 13 males; mean age 63.12 years) underwent the RTM after anterograde reopening dysfunctional biliary stent failure. The inclusion criteria were as follows: (I) age between 18 and 85 years, (II) preoperative imaging showing obvious biliary stent obstruction and bile duct dilation, (III) safe percutaneous puncture access, and (IV) difficulty or failure of the anterograde catheter guide wire in entering the dysfunctional biliary stent. The exclusion criteria were (I) a platelet count ≤25×109/L or prothrombin time >25 s and (II) severe cardiopulmonary dysfunction. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (No. 2013-ky-019). Individual consent for this retrospective analysis was waived. The workflow is shown in Figure 1.
Procedure
The patient laid supine on a digital subtraction angiography (DSA) table (Axiom Artis Zee, Siemens Healthineers, IN, USA). Satisfactory anesthesia was performed 10 minutes before the procedure via local injection with 2% lidocaine (5–10 mL) and intravenous injection with 10 mg of dezocine. A 21-G Chiba needle [percutaneous transhepatic cholangiogram (PTC), Cook Medical] was used to puncture the peripheral dilated bile duct under the guidance of ultrasound and fluoroscopy After cholangiography was performed to understand the biliary tree anatomy, a 0.014-inch platinum guide wire was introduced through the Chiba needle, and a 6-F dilator sheath was introduced along the platinum guidewire to the bile duct. A 0.035-inch guide wire and an 8-F single bend catheter (Terumo Medical Corporation, MD, USA) were introduced into the bile duct after withdrawal of the 6-F dilator sheath. The 8-F catheter was passed through the outer side of the dysfunctional stent to the narrowest segment, and the 0.035-inch guide wire was inserted into the dysfunctional stent through the stent mesh. The guide wire was then manipulated in retrograde fashion from the middle part into the upper part and finally entered into the dilated bile duct segment. The 8-F catheter was retracted to the upper segment of the bile duct expansion section, and the 6-F gooseneck catheter was introduced. Three ring gooseneck catheters were opened, and the head end of the retrograde segment of the guide wire was grasped and gradually pulled out of the body. A 5-F Kumpe (KMP) (Cook Medical, IN, USA) catheter was introduced along the retrograde guide wire, and then an antegrade manipulation access was established. The 5-F catheter was passed through the obstructive segment of the bile duct with 0.035-inch guide wire manipulation. After the reinforcing guidewire (Amplatz, Boston Scientific, IL, USA) was exchanged, safe percutaneous access was established, through which advanced forceps biopsy, 125I strand brachytherapy, biliary radiofrequency ablation, and photodynamic therapy could be performed according to the treatment plan (Figures 2,3). Finally, biliary external-internal drainage or stent reimplantation was performed to decrease high bile duct pressure.


Definitions
Technical success was defined as the successful reopening of the dysfunctional biliary stent using the RTM. Clinical success was defined as a decrease of at least 50% in total bilirubin (TBIL) levels 1 week after the RTM was administered. Postoperative patients underwent imaging evaluation at an interval of 1–2 months to examine tumor control and stent patency. The radiation dose was collected in the irradiation monitoring module of the DSA system. Pretreatment levels of TBIL, direct bilirubin (DB), alanine aminotransferase (ALT), carbohydrate antigen-199 (CA-199), and albumin (ALB) were compared with those taken 1 month after the RTM was applied. Complications were evaluated according to the Society of Interventional Radiology (SIR) Standards of Practice Committee classification on percutaneous hepatobiliary interventions (10). Minor complications were defined as no therapy and no consequence (grade 1) or overnight observation only, no therapy, and no consequence (grade 2). Major complications were defined as requiring therapy and hospitalization (48 hours, grade 3), permanent adverse sequelae (grade 4), or death (grade 5).
Statistical analysis
All statistical analyses were performed using SPSS 26.0 (IBM Corp., USA). Numerical data are expressed as the mean ± standard deviation. Paired t-tests were used to compare the same indices before and after the procedure. P values <0.05 were considered statistically significant.
Results
General characteristics
Among all 25 patients, the primary diagnoses were cholangiocarcinoma (n=12), pancreatic head carcinoma (n=11), and other (n=2), and the local maximum tumor diameter was 33.90±8.10 mm (range, 21.90–55.50 mm). More detailed information is listed in Table 1. The RTM was successfully performed to reopen the occluded stent in all patients, representing a technical success rate of 100%. An 85-year-old patient with pancreatic cancer whose TBIL level was 188.8 µmol/L before the operation underwent forceps biopsy during the operation and experienced bile duct bleeding after the operation, which was 100.5 µmol/L 1 week after the operation with a decrease of 46.77%. The patient was defined as experiencing technical failure. Other patients’ TBIL level decreased by 74.78% (range, 52.8–86.8%), representing a technical success rate of 96%. The mean irradiation dose and procedure times were 774.07±330.80 (range, 408.30–1,808.10) mGy and 45.16±9.48 (range, 36.50–76.00) min, respectively. The median overall survival (OS) was 10.73 months [95% confidence interval (CI): 9.37–12.09]. The levels of TBIL, DB, ALT, CA-199, and ALB pre- and posttreatment are listed in Table 2.
Table 1
| Characteristic | Value |
|---|---|
| Total number | 25 |
| Age (year), mean ± SD (range) | 63.12±12.20 (39.00–85.00) |
| Sex, n (%) | |
| Male | 13 (52.00) |
| Female | 12 (48.00) |
| Primary diagnosis, n (%) | |
| Cholangiocarcinoma | 12 (48.00) |
| Pancreatic head cancer | 11 (44.00) |
| Others | 2 (8.00) |
| Clinical stage, n (%) | |
| Stage III | 7 (28.00) |
| Stage IV | 18 (72.00) |
| Obstruction length (mm), mean ± SD (range) | 30.87±7.73 (13.00–43.90) |
| Local maximum tumor diameter (mm), mean ± SD (range) | 33.90±8.10 (21.90–55.50) |
| Previous noncovered stent type, n (%) | |
| 10 mm × 50 mm | 12 (48.00) |
| 10 mm × 60 mm | 7 (28.00) |
| 8 mm × 50 mm | 5 (20.00) |
| Time to occlusion (month), mean ± SD (range) | 6.55±2.49 (3.50–11.40) |
| Irradiation dose (mGy), mean ± SD (range) | 774.07±330.80 (408.30–1,808.10) |
| Procedure time (min), mean ± SD (range) | 45.16±9.48 (36.50–76.00) |
| Technical success, n (%) | 25 (100) |
| Clinical success, n (%) | 24 (96.00) |
| Complications, n (%) | 2 (8.00) |
| Local tumor control treatment, n (%) | |
| Intraluminal brachytherapy | 11 (44.00) |
| Biliary radiofrequency ablation | 6 (24.00) |
| Photodynamic therapy | 5 (20.00) |
| No treatment | 3 (12.00) |
| Systemic treatment, n (%) | 13 (52.00) |
| Overall survival (month), median (95% CI) | 10.73 (9.37–12.09) |
| Patient status, n (%) | |
| Alive | 9 (36.00) |
| dead | 16 (64.00) |
| Causes of death, n (%) | |
| Tumor cachexia | 8 (32.00) |
| Sudden myocardial infarction | 1 (4.00) |
SD, standard deviation; mGy, milligray; CI, confidence interval.
Table 2
| Characteristics | Pretreatment | Posttreatment (1 month) |
P value |
|---|---|---|---|
| Total bilirubin (μmol/L) | 189.47±59.20 | 44.65±16.12 | <0.001 |
| Direct bilirubin (μmol/L) | 144.21±55.83 | 27.95±13.86 | <0.001 |
| Alanine aminotransferase (U/L) | 89.62±30.85 | 49.44±14.25 | <0.001 |
| Albumin (g/L) | 36.32±2.05 | 40.22±1.95 | <0.001 |
| Carbohydrate antigen-199 (U/mL) | 584.59±269.82 | 176.76±100.68 | <0.001 |
Numerical data are expressed as the mean ± standard deviation. P values are the comparison of the laboratory indices at 1-month postoperation versus those at preoperation, with P<0.05 being significant.
Two patients (8%) experienced complications, one with acute pancreatitis and one with intrabiliary bleeding, with the former experiencing abdominal pain accompanied by an increased amylase level (2,438 U/L). Fasting, nutritional support, acid suppression, antibiotics, and inhibition of pancreatic secretion were administered, and the patient’s symptoms significantly improved, with the amylase level returning to normal within 5 days. The latter patient had intermittent, recurrent bile duct bleeding (20–25 mL/day) and low fever. The patient refused hepatic arteriography and underwent anti-inflammatory and hemostatic treatment for 1 week, after which all issues were resolved.
Discussion
Due to pancreatic head cancer, cholangiocarcinoma, or metastatic cancers, when the bile duct is invaded, there is increased pressure in the bile duct above the obstruction, ultimately leading to bile reflux into the bloodstream. Patients may experience progressive bilirubin elevation or even bilirubinemia fever (11). Considering that the vast majority of patients are in the late tumor stage, surgical intervention is difficult. Due to its minimally invasive and efficient nature, noncovered BSP through PTC drainage or endoscopic retrograde cholangiopancreatography access is the preferred option for relieving bile duct pressure (2).
Poor local tumor control, continuous granulation tissue proliferation, and the reflux of gastrointestinal contents into the bile duct forming blockages may all lead to stent dysfunction (12). In such circumstances, alleviating biliary obstruction can be challenging. At this point, the bile duct above the dysfunctional stent is severely dilated, and the stent is suspended inside the dilated bile duct. After a second percutaneous puncture, entering the catheter and guide wire into the dysfunctional stent and normal lumen becomes technically problematic. Of course, the vast majority of reopening procedures can be completed through precise catheter and guidewire manipulation, but there are always some patients in clinical practice for whom this does not succeed, especially when inexperienced doctors are involved. In such situations, external biliary drainage should be performed for a few days prior to reduce the biliary pressure, and then the expanded bile duct may be retracted before further reopening of the dysfunctional biliary stent; however, this will inevitably delay further minimally invasive treatment in the bile duct. To solve this issue, our center developed the RTM to reopen dysfunctional stents through retrograde manipulation. To the best of our knowledge, there have been no previous reports on this method.
In this study, the success rate of the method was 100%, with 8% being early applications, supporting the safety and feasibility of the technical solution, as these results are equivalent to the relevant technical indicators reported in previous studies on biliary stenting (13-15). One patient experienced clinical failure due to intermittent bleeding (grade 3) after dysfunctional stent opening, which resulted in a bilirubin reduction rate of 46.77%, and being less than 50%, was defined as clinical failure. The levels of TBIL, DBIL, and ALT significantly decreased, while that of ALB increased, indicating that reopening biliary obstruction can still achieve good clinical efficacy.
The RTM procedure is performed as follows: (I) the third level of the dilated bile duct is punctured, with the puncture path being at an angle of approximately 120° to the target bile duct so that the puncture path is smooth and convenient for subsequent manipulation. (II) Satisfactory local anesthesia is administered, which is critical to ensuring good patient cooperation. (III) Real-time ultrasound is used to aid in puncturing the target bile duct under fluoroscopy. (IV) The guide wire of the 8-F catheter then runs clockwise to the most severe obstruction of the stent and then enters the stent starting with the end to allow for a relatively smooth retrograde stent placement. (V) A skilled assistant operates the gooseneck catheter with gentle movements to avoid shifting of the dysfunctional stent. (VI) Appropriate catheter and wire guiding techniques are proficiently performed to improve the success rate of applying the related equipment and to reduce radiation doses.
Despite the outcomes achieved, some limitations to this study should be mentioned. The single-center nature, small sample, and lack of controls represent limits to the study design. Meanwhile, the use of gooseneck catheters can may involve increased clinical diagnosis and treatment costs. In summary, this study is the first to validate the RTM for the reopening of dysfunctional biliary stents. We aim to gain more experience in this method in the future and hope that other centers will adopt it in their clinical practice.
Conclusions
Our findings suggest that the RTM is an effective and safe treatment for patients who require reopening of dysfunctional biliary stents.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1030/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1030/coif). All authors report consulting fees from the American Medical Certification Association (AMCA) and funding from the Henan Province Science and Method Research Project (No. 232102311132). The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu X, Li J, Wu J, Zhu R, Ji W. A Systematic Review and Meta-analysis of Intraluminal Brachytherapy Versus Stent Alone in the Treatment of Malignant Obstructive Jaundice. Cardiovasc Intervent Radiol 2018;41:206-17. [Crossref] [PubMed]
- Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy 2018;50:910-30. [Crossref] [PubMed]
- Almadi MA, Barkun JS, Barkun AN. Stenting in Malignant Biliary Obstruction. Gastrointest Endosc Clin N Am 2015;25:691-711. [Crossref] [PubMed]
- Shabunin AV, Tavobilov MM, Lebedev SS, Karpov AA. Mechanisms and prevention of biliary stent occlusion. Khirurgiia (Mosk) 2020;70-5. [Crossref] [PubMed]
- Shiomi H, Matsumoto K, Isayama H. Management of acute cholangitis as a result of occlusion from a self-expandable metallic stent in patients with malignant distal and hilar biliary obstructions. Dig Endosc 2017;29:88-93. [Crossref] [PubMed]
- Kang H, Han SY, Cho JH, Kim EJ, Kim DU, Yang JK, Jeon S, Park G, Lee TH. Efficacy and safety of temperature-controlled intraductal radiofrequency ablation in advanced malignant hilar biliary obstruction: A pilot multicenter randomized comparative trial. J Hepatobiliary Pancreat Sci 2022;29:469-78. [Crossref] [PubMed]
- Kolarikova M, Hosikova B, Dilenko H, Barton-Tomankova K, Valkova L, Bajgar R, Malina L, Kolarova H. Photodynamic therapy: Innovative approaches for antibacterial and anticancer treatments. Med Res Rev 2023;43:717-74. [Crossref] [PubMed]
- Jiao D, Xu K, Mukhiya G, Liu Y, Wu K, Li Z, Ren J, Han X. Brachytherapy Drainage Catheter and Chemotherapy for Unresectable Pancreatic Carcinoma Combined with Obstructive Jaundice. Front Oncol 2022;12:941336. [Crossref] [PubMed]
- Sejpal D. Advancements in biliary stenting. J Clin Gastroenterol 2012;46:191-6. [Crossref] [PubMed]
- Saad WE, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol 2010;21:789-95. [Crossref] [PubMed]
- Ramchandani M, Lakhtakia S, Costamagna G, Tringali A, Püspöek A, Tribl B, et al. Fully Covered Self-Expanding Metal Stent vs Multiple Plastic Stents to Treat Benign Biliary Strictures Secondary to Chronic Pancreatitis: A Multicenter Randomized Trial. Gastroenterology 2021;161:185-95. [Crossref] [PubMed]
- Nunes TF, Tibana TK, de Andrade GHV, Levigard RB, Nogueira FD, Szejnfeld D. Percutaneous solutions for biliary stent dysfunction: pictorial essay. Radiol Bras 2021;54:43-8. [Crossref] [PubMed]
- Mandai K, Shinomiya R, Uno K, Yasuda K. Transpapillary biliary drainage using a long plastic stent: Preventing early stent dysfunction in pancreatic cancer with duodenal invasion. J Hepatobiliary Pancreat Sci 2022;29:e52-3. [Crossref] [PubMed]
- Takeda T, Sasaki T, Yamada Y, Okamoto T, Mie T, Furukawa T, Kasuga A, Matsuyama M, Ozaka M, Sasahira N. Long-term outcomes of duckbill-type anti-reflux metal stents versus conventional covered metal stents in reinterventions after covered biliary metal stent dysfunction in unresectable pancreatic cancer. Surg Endosc 2023;37:3498-506. [Crossref] [PubMed]
- Furukawa K, Onda S, Hamura R, Taniai T, Marukuchi R, Shiba H, Tsukinaga S, Sumiyama K, Yanaga K. Predictive Factors and Surgical Outcomes of Stent Dysfunction After Preoperative Endoscopic Biliary Stenting in Patients Who Underwent Pancreaticoduodenectomy. J Laparoendosc Adv Surg Tech A 2020;30:256-9. [Crossref] [PubMed]