Prenatal diagnosis of hemimegalencephaly via transabdominal and transvaginal ultrasonography: a case description
Introduction
Hemimegalencephaly (HME) is a rare congenital brain developmental malformation. The main clinical manifestations are intractable epilepsy and neuromotor developmental delay (1), but its pathogenesis is not yet fully understood. According to relevant studies, the occurrence of HME is closely related to gene mutations involved in the PI3K/AKT/mTOR signaling pathway (2,3). Cerebral hemispherectomy is a common treatment method to control epileptic seizures. Although it can relieve symptoms, the prognosis is poor (4). Based on typical ultrasound manifestations, prenatal ultrasound can diagnose HME, enabling early diagnosis, early intervention, and early treatment.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
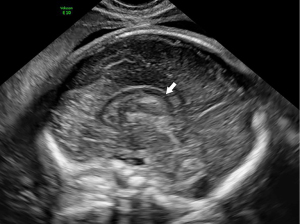
A 29-year-old pregnant woman, G1P0, was seen at the Department of Ultrasound diagnosis at a gestational age of 23 weeks and 6 days. There was no family history of known genetic variants. A Voluson E10 ultrasound diagnostic instrument (GE HealthCare) was used to conducted transabdominal and transvaginal ultrasound and examine the fetal brain. The transabdominal examination results showed that the fetal skull was intact, the biparietal diameter and head circumference were greater than the corresponding gestational age, and the standard deviation of the Z-score was greater than 6. The transverse section of the lateral ventricle and cerebellum showed asymmetry between the left and right cerebral hemispheres. There was a marked ventricular asymmetry with an atrial diameter of 12.5 mm at the left and one of 6.2 mm at the right. The midline structure of the brain had shifted to the right. The cerebral cortex on the left side of the brain was significantly thicker than the right side, and the echo was higher than the right side. The left Sylvian fissure had widened and straightened. The left cerebellar hemisphere was larger than that of the right, and the cistern of the posterior cranial fossa was visible, but there was no significant widening (Figures 1,2). During transvaginal ultrasound, no anomalies were detected in the corpus callosum in the sagittal view (Figure 3). The pericallosal artery was intact and had a normal shape (Figure 4). Ultrasound findings suggested the presence of fetal HME. On the same day, the diagnosis of fetal craniocerebral magnetic resonance imaging (MRI) suggested the possibility of HME brain malformation (Figures 5,6).




Discussion
HME is a rare congenital brain developmental malformation, which is characterized by hamartomatous hyperplasia in all or part of one side of the cerebral hemisphere, with the main manifestations being intractable epilepsy and neuromotor retardation. HME accounts for 1–14% of all cases of cortical dysplasia (1). At present, its pathogenesis is not completely clear. According to the relevant research, HME is classified as a focal, nonneoplastic failure of neuronal/glial proliferation. As such, there is a unilateral overproduction of neurons and glia, which leads to an increased volume of the affected cerebral hemisphere (5). Scarabello et al. (6) demonstrated there to be a significant correlation between HME and ganglion eminence anomalies during embryonic and early fetal life. Other studies suggest that the occurrence of HME is closely related to the gene mutations involved in the PI3K/AKT/mTOR signaling pathway (2,3).
Clinically, HME is mainly divided into three types. The first type, isolated, is the most common type and only involves all or part of the cerebral hemisphere on one side, without any other physical abnormalities. The second type, syndromic, can be complicated with many types of neurocutaneous syndrome, such as tuberous sclerosis, cerebral facial hemangiomatosis, and neurofibromatosis (7). The third type, complete, is characterized by involvement of the ipsilateral cerebellum, brain stem, and the unilateral cerebral hemispheres (8). The fetus described in this case report had complete HME, for which the only possible long-term treatment is hemispherectomy to control seizures, which can relieve symptoms, but still produces a poor prognosis (4). In this case, the parents of the fetus decided to terminate the pregnancy at the 25th week. No other obvious abnormalities (e.g., limb hypertrophy, hemangioma, melanoma) were found in the fetal limbs or skin after induced labor.
In the relevant literature, there are few case reports of MRI used in the prenatal diagnosis of HME, with those diagnosed being mainly newborns and children (9-11). MRI should be considered only in cases where unilateral ventriculomegaly is diagnosed on prenatal ultrasound, especially when associated with any suspicion of asymmetry or midline shift (5). Shah et al. (12) showed that in utero diagnosis of HME with real-time ultrasound is very rare. There are intracranial imaging markers in the ultrasound to aid in the probable diagnosis that can be complemented by MRI evaluation of the brain to confirm the diagnosis. Ultrasound is not only the most important imaging method for prenatal malformation screening but also the primary means of nervous system screening. Alvarez et al. (13) demonstrated that early second trimester prenatal ultrasound diagnosis of HME is possible and reliable. According to the typical ultrasound findings, prenatal ultrasound can indicate the diagnosis of HME. The typical HME ultrasonographic findings are as follows: (I) the left and right cerebral hemispheres are asymmetrical, the affected side is significantly larger than the contralateral side, and the ipsilateral ventricle is broadened; (II) the cerebral cortex of the affected side is thicker than that of the contralateral side, the sulcus is shallow, and the lateral fissure is widened and flattened; (III) the midline structure of the brain appears shifted to the opposite side; (IV) in the complete type, the cerebellar hemisphere and brain stem of the ipsilateral cerebellum are enlarged. In this case, the combined examination of the fetal intracranial structure via transabdominal and transvaginal ultrasound was consistent with the above-described typical ultrasonographic findings. HME can be distinguished from unilateral hydrocephalus, porencephaly, cerebral hemorrhage, and congenital brain tumors. Unilateral hydrocephalus manifests as focal lateral ventricular enlargement rather than global ventricular enlargement. Porencephaly similarly involves focal ex vacuo dilation of a single ventricle and presents with cerebral atrophy rather than cerebral overgrowth. Hemorrhagic and congenital tumors (e.g., neuroectodermal and astrocytic tumors) can present as round masses, similar to the focal white echoes in HME but are associated with ipsilateral ventricular compression rather than ventricular enlargement (14).
HME can be diagnosed on fetal ultrasound from 24 weeks of gestation according to the typical prenatal ultrasonographic features of HME. However, the intrauterine diagnosis of HME should also include multidisciplinary diagnosis and treatment to provide the best prenatal diagnosis, consultation, and treatment.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1546/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wojtowicz A, Duczkowska A, Huras H. A rare case of hemimegalencephaly diagnosed prenatally. Ginekol Pol 2022;93:677-8. [Crossref] [PubMed]
- Severino M, Geraldo AF, Utz N, Tortora D, Pogledic I, Klonowski W, Triulzi F, Arrigoni F, Mankad K, Leventer RJ, Mancini GMS, Barkovich JA, Lequin MH, Rossi A. Definitions and classification of malformations of cortical development: practical guidelines. Brain 2020;143:2874-94. [Crossref] [PubMed]
- Garcia CAB, Carvalho SCS, Yang X, Ball LL, George RD, James KN, Stanley V, Breuss MW, Thomé U, Santos MV, Saggioro FP, Neder Serafini L, Silva WA Jr, Gleeson JG, Machado HR. mTOR pathway somatic variants and the molecular pathogenesis of hemimegalencephaly. Epilepsia Open 2020;5:97-106. [Crossref] [PubMed]
- Lang SS, Goldberg E, Zarnow D, Johnson MP, Storm PB, Heuer GG. Prenatal diagnosis of hemimegalencephaly. World Neurosurg 2014;82:241.e5-8. [Crossref] [PubMed]
- Williams F, Griffiths PD. The diagnosis of hemimegalencephaly using in utero MRI. Clin Radiol 2014;69:e291-7. [Crossref] [PubMed]
- Scarabello M, Righini A, Severino M, Pinelli L, Parazzini C, Scola E, Palumbo G, Di Maurizio M, D'Errico I, Rossi A, Triulzi F, Griffiths PD. Ganglionic Eminence Anomalies and Coexisting Cerebral Developmental Anomalies on Fetal MR Imaging: Multicenter-Based Review of 60 Cases. AJNR Am J Neuroradiol 2021;42:1151-6. [Crossref] [PubMed]
- Serletis D, MacDonald C, Xu Q, Kazina CJ, Dakshinamurti S, Marin S, Del Bigio MR. Hemispherectomy for hemimegalencephaly in a 6.5-week-old infant with tuberous sclerosis complex. Childs Nerv Syst 2022;38:1415-9. [Crossref] [PubMed]
- Jaiswal V, Hanif M, Sarfraz Z, Nepal G, Naz S, Mukherjee D, Ruxmohan S. Hemimegalencephaly: A rare congenital malformation of cortical development. Clin Case Rep 2021;9:e05238. [Crossref] [PubMed]
- Sepulveda W, Sepulveda F, Schonstedt V, Stern J, Diaz-Serani R. NEUROIMAGING FINDINGS IN FETAL HEMIMEGALENCEPHALY: CASE STUDY AND REVIEW. Fetal Diagn Ther 2023; Epub ahead of print. [Crossref]
- Balaji R, Kesavadas C, Ramachandran K, Nayak SD, Priyakumari T. Longitudinal CT and MR appearances of hemimegalencephaly in a patient with tuberous sclerosis. Childs Nerv Syst 2008;24:397-401. [Crossref] [PubMed]
- Flores-Sarnat L. Hemimegalencephaly syndrome. Handb Clin Neurol 2008;87:153-76. [Crossref] [PubMed]
- Shah A, Bharathi N, Reddy T. A Case Report on Prenatal Diagnosis of Evolving Cortical Malformations: A Rare Ultrasound Marker. J Fetal Med 2023;10:085-92.
- Alvarez RM, García-Díaz L, Márquez J, Fajardo M, Rivas E, García-Lozano JC, Antiñolo G. Hemimegalencephaly: prenatal diagnosis and outcome. Fetal Diagn Ther 2011;30:234-8. [Crossref] [PubMed]
- Reis J 3rd, Gill G, Voci S, Almast J. Hemimegalencephaly. Ultrasound Q 2011;27:135-7. [Crossref] [PubMed]