
Successful surgical treatment of a sacral hemangioma causing cauda equina dysfunction: a case description
Introduction
Vertebral hemangioma (VH) is relatively common, occurring in 10% to 12% of the general population (1), and although benign, it is actually a vascular malformation (2). Most VHs are latent [Enneking stage 1 (st.1)] and do not require specific treatment, with only 1% of VHs become active and symptomatic, approximately 45% of which become aggressive and extend into the spinal canal and/or paravertebral space, leading to neurological dysfunction (Enneking st.3) (3). Due to the rarity of aggressive VH cases, the diagnosis and treatment options for these cases remain controversial and problematic. In 2010, Acosta et al. (4) reported that 10 patients with VHs underwent surgical treatment. However, surgical treatment of invasive sacral hemangioma causing cauda equina syndrome with good results has not been reported.
Case presentation
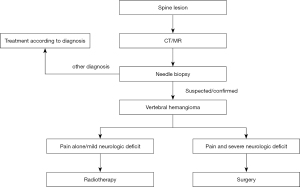
For patients with invasive spinal hemangioma accompanied by neurological dysfunction, our center has a set of diagnostic and treatment procedures, as shown in Figure 1.
Clinical history
The patient was a 61-year-old man who had lumbosacral pain with weakness in both lower extremities for 2 months, numbness in the back of both lower extremities, and difficulty defecating and urinating. No treatment was provided in other medical facilities. The patient had no family history of genetic or similar diseases.
The physical examination showed lumbosacral tenderness, hypoesthesia in the saddle area, hypoesthesia in the posterior lower limbs and dorsolateral feet, hypoesthesia in the hind heels, bilateral weak plantar flexion (grade 3), no ankle reflex, rectal prolapse, anal sphincter muscle strength weakness, and no anal reflexes. All procedures performed in this study were in accordance with the ethical standards of the Peking University First Hospital research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Imaging
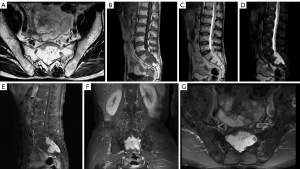
On magnetic resonance imaging (MRI), irregular epidural signals with low T1-weighted imaging (T1WI), high T2-weighted imaging (T2WI), and high fat-suppression T2-weighted imaging (fsT2WI) could be seen in the second sacral vertebra and the first to third sacral vertebrae of the spinal canal. The size of lesion was approximately 5.7 cm × 3.4 cm × 6.2 cm, and there was obvious enhancement on the enhanced scan. A lesion was observed growing along the sacral foramen in a slit-like manner. The bones of the posterior margin of the sacral vertebra 1 were compressed and thin, and only the normal bone of the anterior margin of the second sacral vertebra remained (Figure 2).
Fine needle aspiration pathological biopsy
The sacral mass was found composed of dead bone and bone marrow tissue with active local hyperplasia, vascular structures of different sizes, and uneven wall thickness and lumen size, thus suggesting vascular malformation.
Diagnosis
The ultimate diagnose was a sacral hemangioma.
Surgery
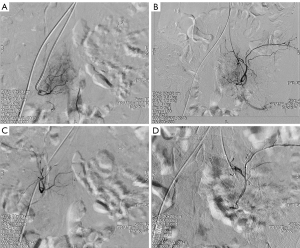
After discussion in the Department of Bone Oncology, Neurosurgery, and Interventional Vascular Surgery, it was decided to perform surgical treatment because the tumor had caused cauda equina nerve function loss but was otherwise benign and had not invaded the surrounding soft tissues. Because preoperative pathologic puncture indicated the possibility of a hemangioma, interventional embolization of local blood supply vessels of the tumor was performed before surgery to avoid massive bleeding during the operation. The local tumor blood supply before and after interventional surgery is shown in the Figure 3. After general anesthesia, the lesion was located with the patient in the prone position, and the lumbosacral posterior median incision was approximately 10 cm long. The L5–S3 spinous process and laminae were exposed. A bone knife and lamina rongeur were used to remove part of L5 lamina and excise the S1–S3 laminae. Red soft solid masses were found in the vertebral canal, which surrounded the sacral nerve root and were attached to the nerve root. The tumor tissue was carefully separated, and the nerve root compression was relieved. The bone was deeply exposed and eroded, and the surface of part of the bone was rough. The bone surface was treated with a scraping spoon and bipolar electrocoagulation. Tumor tissue resection and nerve root lysis were satisfactory, the wound was rinsed, a hemostatic sponge was placed in the wound, a drainage tube was placed, and the incision was closed. The postoperative specimens are shown in Figure 4.
Pathology
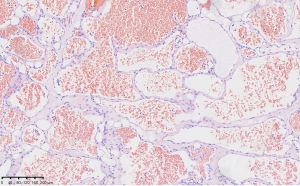
Examination of the sacral lesions revealed that the fibrous adipocytes were lined with flat endothelial cells and contained a large number of red blood cells. The vascular tumors tended to be hemangiomas. An image of the postoperative hematoxylin and eosin (HE) staining of the tumor tissue under microscopy is shown in Figure 5.
Results and follow-up
At 1 month after surgery, the symptoms of lumbosacral pain were significantly relieved, the urinary tube was pulled out, and urination function was gradually restored. Bowel function was slightly restored, but manual assistance was still needed. The sensation in the saddle area and both lower limbs gradually recovered. The plantar flexor strength of the foot was restored to level 4 (+). The ankle reflex was weak, the anal reflex was restored, and the sphincter muscle strength was enhanced. As of this writing, the wound is healing well.
Discussion
The most common tumors in the cauda equina are mucinous ependymoma (90%) and schwannoma, while other less common tumors include paraganglioma, intradural metastasis, hemangioblastoma, and ganglioglioma (5-11). Typically, hemangiomas are mainly located in the vertebrae, accounting for approximately 5% of all spinal vascular lesions, while only approximately 3% are found in the spinal canal with clear boundaries. However, invasive hemangiomas in the sacral vertebrae and that invade the cauda equina nerve root to cause cauda equina syndrome are very rare. In our case, intraoperative hemangioma was clearly found to be surrounding the adherent nerve roots, cause erosion of part of the bone, but the soft tissue around the sacrum was normal, as indicated on MRI. Recent studies have shown that typical cauda equina neurohemangioma is associated with severe neurological dysfunction, and the recovery rate of neurological function after surgical decompression is approximately 100% (3). This was also the approximate rate in our case. However, researchers have also reported cases of tumor recurrence after surgery for this type of aggressive hemangioma (12). In addition, there are some reports that surgical treatment combined with postoperative radiotherapy can reduce the probability of recurrence (13). We believe that the choice of treatment should be based on the patient’s tumor invasion, symptoms, and circumstances. Of course, there are many other treatment options for invasive hemangiomas of the spine that have shown good clinical results, such as radiotherapy alone, vertebroplasty, direct alcohol injection, and surgical decompression with or without radiotherapy (10). In our case, the patient already had symptoms of neurological impairment, so surgery was the preferred treatment; Preoperative MRI examination and further pathologic puncture indicated a hemangioma. Compared with MRI results, the results of pathological puncture are particularly important. The pathological results are the gold standard for our clinical diagnosis and may determine our surgical methods. In order to reduce bleeding, preoperative embolization of tumor blood supply vessels was performed. In addition, we found that the tumor had done little damage to the bone, and a hard bone cortex remained in some areas, so we did not consider vertebroplasty treatment. In addition, the tumor could be completely removed during the operation, so we did not consider complete vertebral resection or postoperative radiotherapy and chemotherapy before first completing a follow-up. If the preoperative pathologic puncture results had indicated that the tumor was malignant, we might have expanded the scope of surgical resection and needed to incorporate postoperative radiotherapy and chemotherapy, rather than simply exfoliating the tumor. In this case, we did not consider the use of alcohol injection. For one, most of the tumors were located in the posterior one-third of the vertebral body and in the spinal canal, and alcohol injection might have damaged the nerves in the spinal canal. For another, our experience with this technology had not yet matured to mastery. However, to prevent tumor recurrence, we burned the tumor and the vertebral interface with alcohol. As of this writing, the patient has shown good recovery after the operation, and there has been no recurrence. Surgery is a relatively effective treatment for invasive sacral hemangioma, but early diagnosis and treatment and postoperative rehabilitation are critical steps for comprehensive treatment, as they can ensure avoiding excessive damage to neurological function and facilitate the recovery of neurological function after surgery. In 2022, Brindisino et al. reported a case of invasive VH with spinal cord compression, which highlighted the importance of rapid clinical diagnosis and treatment as well as postoperative rehabilitation planning with rehabilitation physicians for postoperative neurological recovery (14).
Some limitations to our study should be mentioned. Although the short-term postoperative effect was obvious, long-term follow-up is still needed to observe whether the tumor has recurred and to determine the degree of improvement of nerve function. Generally, patients are highly satisfied with our medical services, as they typically experience a significantly improved quality of life.
Conclusions
Sacral hemangioma with cauda equina involvement is a rare disease that can lead to severe and permanent neurological dysfunction. In the absence of preoperative pathological examination,MRI may be the key examination method for the diagnosis of typical hemangiomas. This is because on MRI, hemangiomas demonstrate certain characteristic features that are conducive to diagnosis. However, in this case, histopathological diagnosis was more important than MRI and could guide further treatment. Even without pathological examination and accurate preoperative diagnosis, surgery may be the preferred treatment for patients with neurological dysfunction.
Acknowledgments
We would like to thank the patient for agreeing to share this case with us as well as the experts from the Medical Imaging Department, Pathology Department, and the Department of Interventional Vascular Surgery of Peking University First Hospital for their attention and guidance in this case.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1493/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg 1993;78:36-45. [Crossref] [PubMed]
- Bremnes RM, Hauge HN, Sagsveen R. Radiotherapy in the treatment of symptomatic vertebral hemangiomas: technical case report. Neurosurgery 1996;39:1054-8. [Crossref] [PubMed]
- Acosta FL Jr, Sanai N, Chi JH, Dowd CF, Chin C, Tihan T, Chou D, Weinstein PR, Ames CP. Comprehensive management of symptomatic and aggressive vertebral hemangiomas. Neurosurg Clin N Am 2008;19:17-29. [Crossref] [PubMed]
- Acosta FL Jr, Sanai N, Cloyd J, Deviren V, Chou D, Ames CP. Treatment of Enneking stage 3 aggressive vertebral hemangiomas with intralesional spondylectomy: report of 10 cases and review of the literature. J Spinal Disord Tech 2011;24:268-75. [Crossref] [PubMed]
- Smith JK, Lury K, Castillo M. Imaging of spinal and spinal cord tumors. Semin Roentgenol 2006;41:274-93. [Crossref] [PubMed]
- Miliaras GC, Kyritsis AP, Polyzoidis KS. Cauda equina paraganglioma: a review. J Neurooncol 2003;65:177-90.
- Boncoeur-Martel MP, Lesort A, Moreau JJ, Labrousse F, Roche I, Bouillet P, Pascaud JL, Dupuy JP. MRI of paraganglioma of the filum terminale. J Comput Assist Tomogr 1996;20:162-5. [Crossref] [PubMed]
- Finn MA, Walker ML. Spinal lipomas: clinical spectrum, embryology, and treatment. Neurosurg Focus 2007;23:E10. [Crossref] [PubMed]
- Koeller KK, Rosenblum RS, Morrison AL. Neoplasms of the spinal cord and filum terminale: radiologic-pathologic correlation. Radiographics 2000;20:1721-49.
- Abul-Kasim K, Thurnher MM, McKeever P, Sundgren PC. Intradural spinal tumors: current classification and MRI features. Neuroradiology 2008;50:301-14. [Crossref] [PubMed]
- Wald JT. Imaging of spine neoplasm. Radiol Clin North Am 2012;50:749-76. [Crossref] [PubMed]
- Wang GX, Chen YQ, Wang Y, Gao CP. Atypical aggressive vertebral hemangioma of the sacrum with postoperative recurrence: A case report. World J Clin Cases 2022;10:12648-53. [Crossref] [PubMed]
- Chen YL, Hu XD, Xu NJ, Jiang WY, Ma WH. Surgical treatment of compressive spinal hemangioma : A case series of three patients and literature review. Orthopade 2018;47:221-7. [Crossref] [PubMed]
- Brindisino F, Scrimitore A, Pennella D, Bruno F, Pellegrino R, Maselli F, Lena F, Giovannico G. Aggressive Vertebral Hemangioma and Spinal Cord Compression: A Particular Direct Access Case of Low Back Pain to Be Managed-A Case Report. Int J Environ Res Public Health 2022;19:13276. [Crossref] [PubMed]



