
Developmental cervical spinal canal size: population reference range
Introduction
The most common indication for cervical spinal surgery in patients aged more than 65 years is spinal canal stenosis leading to myelopathy (1,2). The main factors contributing to cervical spinal stenosis are developmental spinal canal size (i.e., how large is the spinal canal initially) and the degree of superimposed acquired degenerative spinal canal stenosis (3). In addition to spondylotic myelopathy, a developmentally small cervical spinal canal also predisposes to post-traumatic cervical neuropraxia and other types of spinal cord injury (4,5).
Developmental spinal canal narrowing affects the cervical spinal canal diffusely while acquired narrowing, due to disc degeneration, osteophytosis, and facet joint arthrosis affects the disc level, mainly in the mid- to lower cervical spine region (6,7). Less commonly, ossification of the posterior longitudinal ligament (OPLL) may also narrow the cervical spinal canal while diseases, such as dural ectasia, and benign intraspinal tumor may widen the cervical spinal canal.
Spinal canal development is complete by 17 years (6,8,9). Considerable ethnic variation in developmental cervical spinal canal size exists, with Europeans and Americans having larger cervical spinal canals than Asians (10). As such, each population should have a reference range; however, little population data on cervical spinal canal dimensions exists (6,11). To address this problem, this study was designed to develop a population reference interval for developmental cervical spinal canal dimensions for the Hong Kong population.
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Clinical Research Ethics Committee of Joint Chinese University of Hong Kong-New Territories East Cluster (CUHK-NTEC) (No. CRE-2016.527) approved the study. Informed signed consent was provided by all patients. Cervical spinal canal dimensions were prospectively measured on patients undergoing routine neck computed tomography (CT) examinations at the Prince of Wales Hospital, Hong Kong. Study subjects underwent neck CT examinations for a wide range of reasons, with most (60%) for cancer screening (Table 1). Although patients were not healthy at the time of CT examination, use of this data to determine developmental spinal canal size is still valid as systemic diseases incurred after skeletal maturation do not affect spinal canal dimension at the mid-pedicular level (6,12). As our aim was to develop a population reference range, we deliberately did not include or exclude patients with neck symptoms. As developmental spinal canal size is very closely associated with myelopathy and radiculopathy, including only asymptomatic subjects would have introduced bias, favoring patients with developmentally larger spinal canals. Conversely, including only patients with neck symptoms would have introduced bias, favouring patients with developmentally smaller spinal canals (13).
Table 1
| Indication | Subjects (n=522) |
|---|---|
| Cancer | 315 (60%) |
| Trauma | 55 (11%) |
| Nerve palsy | 43 (8%) |
| Thyroid disorder | 29 (6%) |
| Vascular | 24 (5%) |
| Infection | 14 (3%) |
| Salivary gland problem | 11 (2%) |
| GI disorder | 10 (2%) |
| Anemia | 7 (1%) |
| Obstructive sleep apnea | 3 (<1%) |
| Other | 11 (2%) |
Complete count of subjects with respect to reasons for obtaining CT examinations. All vascular indications were performed for assessment of cerebral ischemia. CT, computed tomography.
Subjects
Five hundred and twenty-two patients aged between 20 and 89 years, comprising 256 males (mean 55.0±17.4 years) and 266 females (mean 55.4±17.9 years), were studied. Patients were recruited for seven age groups (e.g., 20–29, 30–39 years, etc.) with at least 30 males and 30 females in each age group. For reliability testing of a continuous variable, a sample size of at least 30 subjects is sufficient (14). Patients with non-Chinese names were not included as well as patients with (I) known spinal disorder such as scoliosis, (II) previous cervical spine surgery, (III) childhood chronic inflammatory illnesses, and (IV) major cervical spine structural abnormality such as cervical vertebral fracture, spinal dysraphism or OPLL on CT assessment. All eligible patients had their height (centimeters) and weight (kilograms) measured before CT examination. The body mass index (BMI) of the study cohort was compared to the BMI scores of the Hong Kong general public obtained for the government’s Centre for Health Protection surveys (ref, Centre for Health protection). No significant difference between the study cohort and the general population (P>0.05) was present, indicating that subject body size was representative of the overall population.
Data collection
CT examinations were acquired on a 64-slice multidetector CT machine with 0.6mm reconstructive resolution. Spinal canal and vertebral body measurements were obtained on data reconstructed in a true axial plane for each vertebra from C3 to C7 (2,610 levels assessed) using ITK-SNAP 3.6.0. The C1 and C2 vertebrae were not included as degenerative spinal canal stenosis is rare in the upper cervical region and these two vertebrae are different morphologically and functionally from the remainder of the cervical spine (11,15). Following optimal image zooming and contrast adjustment, the spinal canal cross-sectional area (CSA), anteroposterior (AP) sagittal diameter, and width as well as the vertebral body CSA, AP sagittal diameter and width were measured at each level manually by one of two operators.
Spinal canal CSA
Spinal canal CSA was measured by demarcating the spinal canal boundary at the inner margin of the cortex of the vertebral body, pedicles, and lamina (Figure 1).
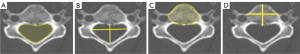
Spinal canal AP sagittal diameter and width
Spinal canal AP sagittal diameter (maximum AP diameter) was measured from the periosteal cortical margin at the posterior aspect of the vertebral body to the periosteal cortical margin of the neural arch at the base of the spinous process (Figure 1). Spinal canal width (maximum mediolateral) was measured between the periosteal cortical margins of both pedicles (Figure 1). Spinal canal AP sagittal diameter is the most reliable developmental canal size measure to differentiate patients at risk of cervical spondylotic myelopathy and cervical cord trauma (5,11).
Vertebral body CSA
Vertebral body CSA was measured by measuring along the cortical outline at the edges of the vertebral body (Figure 1).
Vertebral body AP sagittal diameter and width
Vertebral body AP sagittal diameter was measured from the anterior mid-point to the posterior mid-point of the vertebral body. Vertebral body width was measured from the mid-point of the vertebral wall on the right side to the mid-point of the vertebral wall on the left side (Figure 1).
Spinal canal AP sagittal diameter to width ratio
From these measurements, the spinal canal AP sagittal diameter to width ratio was calculated for each level. This AP sagittal diameter to width ratio is the second most reliable developmental canal size measure to differentiate patients at risk of cervical spondylotic myelopathy and cervical cord trauma (5,11).
Vertebral body AP sagittal diameter to spinal canal AP sagittal diameter ratio
The radiographic Parlov-Torg ratio is a useful radiographic measurement of cervical cord injury risk. However, the CT Parlov-Torg ratio seems to not be as good an injury risk discriminator as either the spinal canal AP sagittal diameter or spinal canal AP sagittal diameter to width ratio (16).
Thresholds representing smallest 25% of population
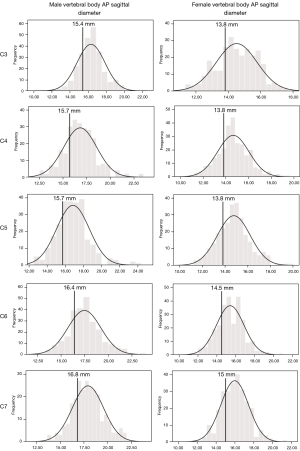
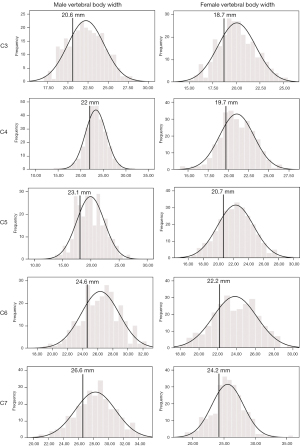
Histograms for males and females were drawn for each spinal canal measure to ensure normal distribution. An arbitrary threshold representing the smallest 25% quartile of the population was used to indicate a developmentally small spinal canal CSA, AP sagittal diameter, or width; vertebral body CSA, AP sagittal diameter, or width; and spinal canal AP sagittal diameter to width ratio.
Reliability
To examine reliability, each of the two operators collected 250 measurements for areal values and 250 measurements for length and AP sagittal diameter on the same subjects blinded to the other’s results. Results were compared for reliability.
Statistical analysis
Spinal dimensions and biometric measurements were collected and analyzed for statistical correlation and significance using the statistical package SPSS version 26.0 (SPSS Inc., Illinois, USA). For inter-rater reliability, intraclass correlation coefficient (ICC) estimates were calculated based on a single-rating (k=2), absolute agreement, two-way random-effects model. Independent 2-sample t-test was used to test for gender differences for all spinal measurements (spinal canal and vertebral body CSA, spinal canal AP sagittal diameter and width). Pearson’s correlation was conducted to measure the associations between clinical measurements (age, height, weight, BMI) and spinal measurements (spinal canal and vertebral body CSA, spinal canal AP sagittal diameter and width). A probability, P value <0.5 was considered statistically significant.
Results
Study subjects
Mean male and female height were 166.6±8.8 and 155.7±6.7 cm, respectively; while mean male and female weight were 64.3±13.3 and 55.7±12.3 kg, respectively. Males were taller (P<0.0001) and heavier (P<0.0001) than females. BMI was similar for both sexes (23.1±4.2 vs. 22.9±4.5 kg/m2, P=0.58). With increasing age, height and weight reduced slightly for both sexes (Table 2).
Table 2
| Variable | Age | Height | Weight | BMI | SC CSA C3–7 |
SC AP sagittal diameter C3–7 | SC width C3–7 |
VB CSA C3–7 |
VB AP sagittal diameter C3–7 | VB width C3–7 |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| Height | −0.46 (<0.001) | |||||||||
| Weight | −0.33 (<0.001) | 0.47 (<0.001) | ||||||||
| BMI | −0.1 (0.1) | −0.07 (0.2) | 0.84 (<0.001) | |||||||
| SC CSA C3–7 | −0.23 (<0.001) | 0.25 (<0.001) | 0.12 (0.05) | 0 (1) | ||||||
| SC AP sagittal diameter C3–7 | −0.13 (0.04) | 0.15 (0.02) | 0.1 (0.1) | 0.04 (0.6) | 0.78 (<0.001) | |||||
| SC width C3–7 | −0.21 (<0.001) | 0.26 (<0.001) | 0.05 (0.4) | −0.08 (0.2) | 0.72 (<0.001) | 0.39 (<0.001) | ||||
| VB CSA C3–7 | 0.43 (<0.001) | 0.17 (0.008) | 0.09 (0.1) | −0.01 (0.9) | −0.01 (0.9) | −0.06 (0.4) | 0.06 (0.3) | |||
| VB AP sagittal diameter C3–7 | 0.48 (<0.001) | 0.03 (0.6) | 0.12 (0.05) | 0.1 (0.1) | −0.1 (0.1) | −0.18 (0.005) | −0.1 (0.1) | 0.75 (<0.001) | ||
| VB width C3–7 | 0.16 (0.01) | 0.06 (0.3) | 0.02 (0.8) | −0.03 (0.6) | 0.4 (<0.001) | 0.16 (0.01) | 0.37 (<0.001) | 0.5 (<0.001) | 0.27 (<0.001) | |
| SC AP sagittal diameter/ width ratio C3–7 |
0.09 (0.2) | −0.1 (0.1) | 0.03 (0.6) | 0.09 (0.1) | 0.14 (0.02) | 0.63 (<0.001) | −0.44 (<0.001) | −0.08 (0.2) | −0.05 (0.4) | −0.13 (0.04) |
| Female | ||||||||||
| Height | −0.4 (<0.001) | |||||||||
| Weight | −0.21 (<0.001) | 0.41 (<0.001) | ||||||||
| BMI | −0.06 (0.4) | 0.02 (0.8) | 0.92 (<0.001) | |||||||
| SC CSA C3–7 | −0.33 (<0.001) | 0.36 (<0.001) | 0.23 (<0.001) | 0.09 (0.2) | ||||||
| SC AP sagittal diameter C3–7 | −0.21 (<0.001) | 0.28 (<0.001) | 0.21 (<0.001) | 0.11 (0.08) | 0.83 (<0.001) | |||||
| SC width C3–7 | −0.24 (<0.001) | 0.35 (<0.001) | 0.21 (<0.001) | 0.07 (0.3) | 0.76 (<0.001) | 0.58 (<0.001) | ||||
| VB CSA C3–7 | 0.46 (<0.001) | 0.07 (0.3) | 0.02 (0.7) | −0.01 (0.9) | 0 (0.9) | −0.14 (0.02) | −0.01 (0.8) | |||
| VB AP sagittal diameter C3–7 | 0.51 (<0.001) | −0.05 (0.4) | 0.03 (0.7) | 0.05 (0.5) | −0.18 (0.003) | −0.22 (<0.001) | −0.08 (0.2) | 0.61 (<0.001) | ||
| VB width C3–7 | 0.17 (0.005) | 0.09 (0.1) | 0.09 (0.1) | 0.05 (0.4) | 0.25 (<0.001) | 0.1 (0.1) | 0.25 (<0.001) | 0.47 (<0.001) | 0.37 (<0.001) | |
| SC AP sagittal diameter/ width ratio C3–7 |
−0.03 (0.6) | 0 (0.9) | 0.05 (0.4) | 0.07 (0.3) | 0.3 (<0.001) | 0.66 (<0.001) | −0.22 (<0.001) | −0.14 (0.02) | −0.18 (0.003) | −0.11 (0.07) |
Correlation between clinical and spinal measurements. Data are presented as Pearson’s correlation coefficient (with P value) for different clinical and spinal measurements. BMI, body mass index; SC, spinal canal; CSA, cross-sectional area; AP, anteroposterior; VB, vertebral body.
Spinal canal CSA
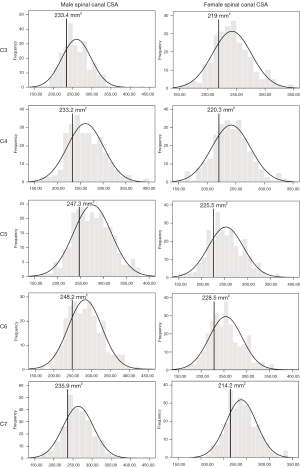
Developmental spinal canal CSA was smallest at C3 for males and at C7 for females, and largest at C6 for both sexes (Table 3). Mean canal CSA at the mid-C5 level was 276±41.5 mm2 in males and 252.6±38.4 mm2 in females (Table 3). Percentage difference between the smallest and largest CSA at C5 was 164% for males and 168% for females. The spinal canal CSA was larger in males at all levels (Table 3). For both sexes, a weak negative correlation existed between age and spinal canal CSA, as well as a weak positive correlation between height and cervical spine CSA (Table 2).
Table 3
| Vertebral level | Spinal canal CSA (mm2) | Spinal canal depth (mm) | Spinal canal width (mm) | Spinal canal depth/width ratio |
|---|---|---|---|---|
| C3 | ||||
| Male | 259.6 (38.6) | 13.83 (1.33) | 23.66 (1.63) | 0.59 (0.07) |
| Female | 242.1 (34) | 13.35 (1.28) | 22.56 (1.56) | 0.59 (0.05) |
| P value | <0.0001 | <0.0001 | <0.0001 | 0.3903 |
| C4 | ||||
| Male | 261.4 (39.6) | 13.35 (1.33) | 26.03 (2.06) | 0.52 (0.06) |
| Female | 241.6 (33.9) | 12.92 (1.18) | 24.47 (1.75) | 0.53 (0.04) |
| P value | <0.0001 | 0.0001 | <0.0001 | 0.0076 |
| C5 | ||||
| Male | 276 (41.5) | 13.75 (1.34) | 27.39 (2.2) | 0.5 (0.05) |
| Female | 252.6 (38.4) | 13.15 (1.28) | 25.72 (1.99) | 0.51 (0.05) |
| P value | <0.0001 | 0.0001 | <0.0001 | 0.0499 |
| C6 | ||||
| Male | 282.1 (44.5) | 14.16 (1.43) | 26.94 (2.31) | 0.53 (0.11) |
| Female | 254 (36.2) | 13.5 (1.39) | 25.6 (1.89) | 0.53 (0.05) |
| P value | <0.0001 | <0.0001 | <0.0001 | 0.3158 |
| C7 | ||||
| Male | 263 (38.3) | 14.21 (1.42) | 25.52 (2.26) | 0.56 (0.09) |
| Female | 237.9 (33.4) | 13.37 (1.29) | 24.42 (2.05) | 0.55 (0.06) |
| P value | <0.0001 | <0.0001 | <0.0001 | 0.0246 |
Data are presented as mean (standard deviation). Mean spinal canal from C3 to C7. CSA, cross-sectional area.
Spinal canal AP sagittal diameter and width
Developmental spinal canal AP sagittal diameter was largest at C7 for males and at C6 for females (Table 3). Mean canal AP sagittal diameter at the mid-C5 level was 13.75±1.34 mm in males and 13.15±1.28 mm in females (Table 3). Percentage difference between the smallest and largest canal AP sagittal diameter at C5 was 83% for males and 82% for females. Overall, spinal canal AP sagittal diameter reduced slightly in both males and females (Table 2). Both sexes had a weak positive correlation between height and spinal canal AP sagittal diameter (Table 2). Spinal canal AP sagittal diameter was larger in males at all levels (Table 3).
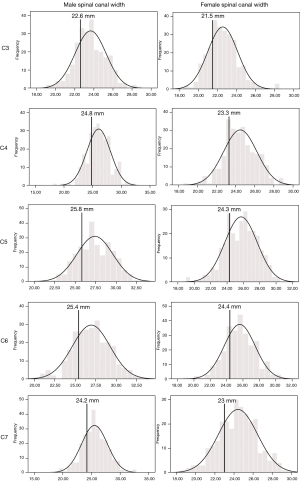
Developmental spinal canal width was largest at C5 and smallest at C3 for both sexes (Table 3). Mean canal width at the mid-C5 level was 27.39±2.2 mm in males and 25.72±1.99 mm in females (Table 3). Percentage difference between the smallest and largest canal width at C5 was 63% for males and 60% for females. At all levels, males had a large spinal canal width than females (Table 3). For both sexes, a highly significant weak positive correlation existed between height and spinal canal width (Table 2).
Vertebral body CSA
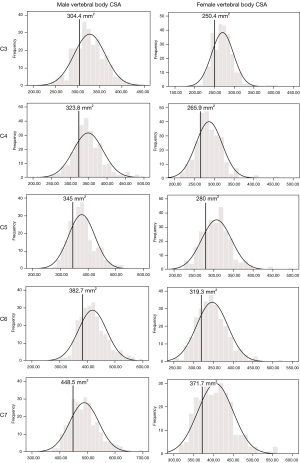
Vertebral body CSA increased gradually from C3 to C7 for both sexes and was significantly larger in males at all levels (Table 4). For both sexes, a highly significant moderate positive correlation existed between age and vertebral body CSA (Table 2). Percentage difference between the smallest and largest vertebral body CSA at C5 was 122% for males and 117% for females. Although males had larger spinal canal CSAs than females, relative to vertebral body CSA, spinal canal CSA was larger in females.
Table 4
| Vertebral level | Vertebral body CSA (mm2) | Vertebral body depth (mm) | Vertebral body width (mm) | Vertebral body depth/spinal canal depth ratio |
|---|---|---|---|---|
| C3 | ||||
| Male | 327.3 (35.1) | 16.31 (1.54) | 22.23 (2.26) | 1.19 (0.18) |
| Female | 270.6 (27.8) | 14.5 (1.26) | 20.06 (2.02) | 1.11 (0.16) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C4 | ||||
| Male | 348.9 (40.5) | 16.54 (1.61) | 23.35 (2.32) | 1.26 (0.2) |
| Female | 287.3 (33.3) | 14.64 (1.38) | 21.04 (2.14) | 1.15 (0.17) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C5 | ||||
| Male | 377.2 (47.6) | 16.81 (1.75) | 24.95 (2.45) | 1.24 (0.21) |
| Female | 307.6 (37.8) | 14.76 (1.44) | 22.32 (2.41) | 1.14 (0.19) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C6 | ||||
| Male | 418.7 (50.9) | 17.46 (1.74) | 26.33 (2.54) | 1.25 (0.2) |
| Female | 346.6 (39.6) | 15.4 (1.46) | 23.84 (2.32) | 1.16 (0.18) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C7 | ||||
| Male | 489 (52.2) | 17.91 (1.65) | 28.27 (2.69) | 1.28 (0.19) |
| Female | 405.1 (44.4) | 15.93 (1.45) | 25.59 (2.54) | 1.21 (0.17) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Data are presented as mean (standard deviation). Mean vertebral body from C3 to C7. CSA, cross-sectional area.
Vertebral body AP sagittal diameter and width
Vertebral body AP sagittal diameter and width CSA increased gradually from C3 to C7 for both sexes and, at all levels, was larger in males (Table 4). There was a highly significant moderate correlation between vertebral body AP sagittal diameter and age in both sexes (Table 2). Percentage difference between the smallest and largest vertebral body AP sagittal diameter at C5 was 83% for males and 82% for females.
Vertebral body width increased gradually from C3 to C7 for both sexes and, at all levels, was larger in males (Table 4). Percentage difference between the smallest and largest vertebral body AP sagittal diameter at C5 was 63% for males and 60% for females.
Spinal canal AP sagittal diameter to width ratio
Spinal canal AP sagittal diameter to width ratio was smallest at C5 for both sexes, tending to increase slightly both in cranial and caudal directions (Table 3).
Vertebral body AP sagittal diameter to spinal canal AP sagittal diameter ratio
Vertebral body AP sagittal diameter to spinal canal AP sagittal diameter ratio was smallest at C3 (Table 4).
Thresholds representing smallest 25% of population
The developmental parameters demarcating the smallest 25% of the population for each vertebral level and gender are shown in Figures 2-8.


Reliability
The ICC for CSA measurements and length measurements were 0.924 and 0.976, respectively, indicating excellent reliability between the two raters.
Discussion
Developmental spinal canal size is measured at the mid-vertebral body level removed from acquired degenerative changes occurring at the discovertebral level. This study concurs with other studies in showing considerable population variation in developmental spinal canal size (11). There was, for example, a 166% spread between the smallest (3SD below mean) and largest (3SD above mean) spinal canal CSA at C5. A developmentally large spinal canal has no known disadvantage. All problems arise from possessing a developmentally small cervical spinal canal. Patients with a developmentally small spinal canal present about 5 years earlier with spondylotic myelopathy and with worse neurological impairment than patients with a developmentally large spinal canal (17). As developmental spinal canal narrowing may also be associated with shorter pedicles and smaller foramina, there may be an increased risk of degenerative foraminal stenosis and radiculopathy. The presence of a small cervical spinal canal may influence the type of decompressive surgery undertaken and have a less favourable post-surgical outcome (1,2). The risk of traumatic cord injury is also increased with a developmentally small spinal canal (4,5). A developmentally small spinal canal may also affect spinal biomechanics potentially accelerating degenerative disease (18).
In line with previous studies, all spinal canal and vertebral dimensions were slightly larger in male than females (11,15). Taller persons had a slightly larger developmental spinal canal CSA, mainly due to an increase in spinal canal width rather than AP sagittal diameter. Weight or BMI did not influence developmental spinal canal size. In addition, this study shows that, with increasing age, vertebral body CSA and AP sagittal diameter (but not width) increases between C3 and C7. As vertebral body size increases, there is a slight reduction in developmental cervical spinal canal CSA with increasing age, especially in females.
Once the population reference range was developed for each level and sex, an arbitrary cut-off of the smallest 25% of the range was taken to present a developmentally small spinal canal. Acquired degenerative changes occurring at the discovertebral level compound a pre-existing developmentally small spinal canal size. The two developmental spinal canal parameters, measured at the mid-vertebral level, which are the best at differentiating between cohorts of patients with and without spinal cord compression are (I) the AP diameter of the cervical spinal canal and (II) the AP to transverse ratio of the cervical spinal canal (5,11). Regarding AP diameter, a developmentally narrow canal has been quoted as an AP diameter of <14 mm (19) or <12 mm (11). Regarding AP to transverse ratio, the risk of cervical myelopathy is greater if this ratio is <0.5 at C4–C6 levels (11). Such parameters fit well with an arbitrary cut-off of the smallest 25% of the population as applied in this study.
Using CT data enables readily acquisition of large volume data, though CT does not allow measurement of spinal cord size. Our aim was to develop a reference range for spinal canal size which is known to vary significantly more than spinal cord size (9,13). As both CT and MRI are cross-sectional modalities, there should be good correlation between CT and MRI measurements, though no such cross-collaborative studies have been performed. Relatively minor selective bias may be present as we studied only a hospital population; however, this bias is likely to be minor as the indications for CT examination are not known to affect developmental cervical spinal canal size.
The potential benefits of knowing a population reference range are considerable. This reference range will allow one to (I) have a reliable benchmark against which to objectively assess an individual patient’s developmental cervical spinal canal size. This could potentially be incorporated into hospital systems to provide normal reference intervals for spinal canal size facilitating decision making. (II) Explore the factors (such as genetic, familial, neonatal, or perinatal) that govern the etiology of a developmentally narrow spinal canal. (III) Enhance predictive models related to the likelihood of spinal cord compression, surgery to surgical outcome. Such predictive models could be applied to occupational settings related to risks associated with high-contact sports or the prolonged wearing of helmets. (IV) Assist in medico-legal issues pertaining to an individual’s pre-disposition to developing spinal canal stenosis or cord compression. (V) Provide a template of using CT databases for developing a reference range in which other populations can easily undertake comparative studies, given that over 5 million neck CT examinations are performed yearly around the world (20) and (VI) increase awareness of developmental spinal canal size as an important measure in the assessment of the cervical spinal canal amongst clinicians and the general population.
There are some limitations to this study. CT, and not MRI measurements, were obtained as large population CT data was more readily available and to include patients attending for cervical spine MRI for suspected neural compression would have skewed data towards a developmentally small spinal canal. Due to difficulty separating the bony cortex from the overlying posterior longitudinal ligament and ligamentum flavum as well as issues related to pulsatility artefact and cerebrospinal fluid pulsation, MRI values tend to be about 8% smaller than CT values (21). Only spinal canal size and not neural foraminal size was evaluated.
Conclusions
A population reference range for developmental cervical spinal canal size was developed. This will allow a more objective quantitative assessment of developmental spinal canal narrowing. Such information is helpful for defining what constitutes a developmentally narrow spinal canal, as well as investigating factors governing the aetiology of developmental spinal canal stenosis, predicting cord injury risk, and surgical outcome.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1395/coif). J.F.G. serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Clinical Research Ethics Committee of Joint CUHK-NTEC (No. CRE-2016.527) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J 2006;6:190S-7S. [Crossref] [PubMed]
- Nagata K, Yoshimura N, Hashizume H, Ishimoto Y, Muraki S, Yamada H, Oka H, Kawaguchi H, Akune T, Tanaka S, Nakamura K, Yoshida M. The prevalence of tandem spinal stenosis and its characteristics in a population-based MRI study: The Wakayama Spine Study. Eur Spine J 2017;26:2529-35. [Crossref] [PubMed]
- Dora C, Wälchli B, Elfering A, Gal I, Weishaupt D, Boos N. The significance of spinal canal dimensions in discriminating symptomatic from asymptomatic disc herniations. Eur Spine J 2002;11:575-81. [Crossref] [PubMed]
- Torg JS, Pavlov H, Genuario SE, Sennett B, Wisneski RJ, Robie BH, Jahre C. Neurapraxia of the cervical spinal cord with transient quadriplegia. J Bone Joint Surg Am 1986;68:1354-70.
- Matsuura P, Waters RL, Adkins RH, Rothman S, Gurbani N, Sie I. Comparison of computerized tomography parameters of the cervical spine in normal control subjects and spinal cord-injured patients. J Bone Joint Surg Am 1989;71:183-8.
- Griffith JF, Huang J, Law SW, Xiao F, Leung JC, Wang D, Shi L. Population reference range for developmental lumbar spinal canal size. Quant Imaging Med Surg 2016;6:671-9. [Crossref] [PubMed]
- Goto S, Umehara J, Aizawa T, Kokubun S. Comparison of cervical spinal canal diameter between younger and elder generations of Japanese. J Orthop Sci 2010;15:97-103. [Crossref] [PubMed]
- Lee HJ, Lee JJ, Hong JT, Kim JT. Quantification of pediatric cervical growth: anatomical changes in the sub-axial spine. J Korean Neurosurg Soc 2015;57:185-91. [Crossref] [PubMed]
- Ulbrich EJ, Schraner C, Boesch C, Hodler J, Busato A, Anderson SE, Eigenheer S, Zimmermann H, Sturzenegger M. Normative MR cervical spinal canal dimensions. Radiology 2014;271:172-82. [Crossref] [PubMed]
- Chazono M, Tanaka T, Kumagae Y, Sai T, Marumo K. Ethnic differences in pedicle and bony spinal canal dimensions calculated from computed tomography of the cervical spine: a review of the English-language literature. Eur Spine J 2012;21:1451-8. [Crossref] [PubMed]
- Toki S, Higashino K, Manabe H, Morimoto M, Sugiura K, Tezuka F, Yamashita K, Takata Y, Maeda T, Sakai T, Yasui N, Sairyo K. Morphometric Analysis of Subaxial Cervical Spine with Myelopathy: A Comparison with the Normal Population. Spine Surg Relat Res 2021;5:34-40. [Crossref] [PubMed]
- Schizas C, Schmit A, Schizas A, Becce F, Kulik G, Pierzchała K. Secular changes of spinal canal dimensions in Western Switzerland: a narrowing epidemic? Spine (Phila Pa 1976) 2014;39:1339-44. [Crossref] [PubMed]
- Griffith JF. Advanced Quantitative Spine Imaging. Semin Musculoskelet Radiol 2020;24:413-27. [Crossref] [PubMed]
- Hobart JC, Cano SJ, Warner TT, Thompson AJ. What sample sizes for reliability and validity studies in neurology? J Neurol 2012;259:2681-94. [Crossref] [PubMed]
- Monier A, Omoumi P, Schizas S, Becce F, Schizas C. Dimensional changes of cervical and lumbar bony spinal canals in one generation in Western Switzerland: a computed tomography study. Eur Spine J 2017;26:345-52. [Crossref] [PubMed]
- Kumar A, Sahu S, Sethi S, Ratre S, Parihar V, Swamy N, Yadav YR. Computerized Tomography-Based Morphometric Analysis of Cervical Spinal Canal in Central Indian Population. J Neurosci Rural Pract 2020;11:274-7. [Crossref] [PubMed]
- Nouri A, Tetreault L, Nori S, Martin AR, Nater A, Fehlings MG. Congenital Cervical Spine Stenosis in a Multicenter Global Cohort of Patients With Degenerative Cervical Myelopathy: An Ambispective Report Based on a Magnetic Resonance Imaging Diagnostic Criterion. Neurosurgery 2018;83:521-8. [Crossref] [PubMed]
- Morishita Y, Naito M, Hymanson H, Miyazaki M, Wu G, Wang JC. The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J 2009;18:877-83. [Crossref] [PubMed]
- Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F. Narrow cervical canal in 1211 asymptomatic healthy subjects: the relationship with spinal cord compression on MRI. Eur Spine J 2016;25:2149-54. [Crossref] [PubMed]
- Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 2009;169:2071-7. [Crossref] [PubMed]
- Grams AE, Gempt J, Förschler A. Comparison of spinal anatomy between 3-Tesla MRI and CT-myelography under healthy and pathological conditions. Surg Radiol Anat 2010;32:581-5. [Crossref] [PubMed]


