
The efficacy of modified contrast-enhanced ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) using Sonazoid in diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis
Introduction
Hepatocellular carcinoma (HCC) accounts for 75–85% of primary liver cancer cases, making it the third most common cause of cancer-related death globally, following lung cancer and colorectal cancer, and ranking fifth in terms of cancer incidence (1). Early diagnosis and aggressive treatment are essential to improve the prognosis of patients with HCC. Currently, HCC is the sole form of cancer that can be noninvasively diagnosed in high-risk individuals using various imaging tests, without the need for pathological findings (2-6). Due to the benefits of pure blood pool imaging, the ability to observe in real-time, and the high level of safety for patients with renal insufficiency and iodine allergy, contrast-enhanced ultrasound (CEUS) has been widely used in clinical diagnostic workups (7). In Europe and Asia, CEUS has been adopted as the primary diagnostic technique for HCC and has gained recognition from numerous national and international professional organizations (2-6). The American College of Radiology (ACR) released the first version of the CEUS Liver Imaging Reporting and Data System (LI-RADS) in 2016, which was subsequently revised in 2017. The algorithm is offered for the diagnosis of patients with a high risk for HCC (8). CEUS LI-RADS categorizes liver nodules based on the probability of HCC occurrence in high-risk patients, ranging from LR-1 (definitely benign) to LR-5 (definitely HCC), and also includes LR-M and LR-TIV classifications. LR-M indicates that the observations are probably or definitely malignant, but they do not exhibit typical features of HCC. LR-TIV represents 100% certainty malignant lesion with tumor in the vein. In recent meta-analyses on CEUS LI-RADS, it was found that LR-5 had a sensitivity of 0.71, 0.69 and specificity of 0.93, 0.93 for diagnosing HCC (9,10). The algorithm showed high and stable specificity. According to the present edition of CEUS LI-RADS (v2017), this categorization is specifically for exclusive blood pool contrast agents and should not be used for CEUS examinations that involve both blood pools and a Kupffer cells agent such as perfluorobutane (Sonazoid; GE Healthcare, Chicago, IL, USA). Furthermore, it is clearly mentioned that the utilization of these contrast agents will be incorporated in the upcoming edition.
Sonazoid is composed of microspheres coated with hydrogenated egg phosphatidyl serine (HEPS) and filled with perfluorobutane gas (PFB) (11). Unlike SonoVue (Bracco, Milan, Italy), it has the capability to be phagocytosed by Kupffer cells within the liver and/or reticuloendothelial cells. Therefore, Sonazoid has been shown to provide a unique Kupffer phase (KP) of hepatic parenchyma in addition to a dynamic vascular phase (12,13). The KP happens 10 minutes following the injection of contrast, and cancerous growths exhibit defects compared to the nearby healthy liver tissue because of the decrease or lack of Kupffer cells (7). The characteristic appearance of HCC on Sonazoid ultrasonography usually involves hyperenhancement during the arterial phase, decreased or unchanged enhancement during the portal or late phase, and the presence of KP defects. This typical pattern is observed in over 97% of HCC cases (14).
It has been found that when Sonazoid was used for CEUS, a longer observation time resulted in more HCCs showing hypoenhancement. Defects in the KP have been observed in certain HCCs that did not exhibit washout during the vascular phase (15). The application of KP is anticipated to enhance the sensitivity of HCC detection in individuals at high risk. Enhancing the sensitivity of HCC diagnosis is crucial in regions where radical surgical removal and local ablation are the primary therapeutic choices for HCC (16). Researchers have recently suggested a modified CEUS LI-RADS LR-5 by replacing the mild and late (≥60 seconds) washout with KP defects as the primary imaging features. They have conducted original studies to investigate the significance of KP in diagnosing HCC (17-24). Currently, numerous meta-analyses have examined the accuracy of contrast-enhanced computed tomography/magnetic resonance imaging (CT/MRI) LI-RADS and CEUS LI-RADS in detecting HCC and other non-HCC malignancies (OM) (9,25-27), whereas other studies have compared the diagnostic performance of different imaging modalities (10). Nevertheless, there is a lack of a comprehensive assessment or meta-analysis to appraise the diagnostic effectiveness of LR-5 in modified CEUS LI-RADS using Sonazoid contrast agent for HCC. We hypothesized that the modified CEUS LI-RADS LR-5 would exhibit significant sensitivity and specificity in diagnosing HCC in high-risk patients. Hence, we conducted a meta-analysis to assess the accuracy of LR-5 in modified CEUS LI-RADS for diagnosing HCC. We present this article in accordance with the PRISMA-DTA reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1184/rc) (25).
Methods
Our research program is registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) with registration number INPLASY202380044.
Search strategy
A thorough and organized search was performed using the databases of PubMed, Embase, the Cochrane Library, and Web of Science until 13 July 2023, without any limitations on language. Table S1 displays the specific search strategies utilized, incorporating search terms like “liver neoplasms”, “LI-RADS”, “CEUS”, and “Sonazoid”.
Eligibility criteria
The study included original research that used Sonazoid contrast agents for CEUS in patients who were at a high risk of HCC. The study aimed to assess the effectiveness of LR-5 in modified CEUS LI-RADS for diagnosing HCC. According to CEUS LI-RADS (v2017), individuals with cirrhosis, chronic infection of hepatitis B virus, and current or previous HCC are considered at a high risk for HCC. Patients under 18 years old, without the mentioned risk factors, with cirrhosis caused by congenital hepatic fibrosis, and with cirrhosis from vascular disorder (e.g., hereditary hemorrhagic telangiectasia, Budd-Chiari syndrome, chronic portal vein occlusion, cardiac congestion, or diffuse nodular regenerative hyperplasia) do not conform to this risk group (8). The definition of Modified CEUS LI-RADS LR-5 includes arterial phase hyperenhancement (APHE) (excluding rim and peripheral discontinuous globular enhancement) and KP defects.
In this study, the literature exclusion criteria consisted of the following: (I) meta-analyses, reviews, evaluations, case studies, correspondences, remarks, and summaries of conferences; (II) research that falls outside the scope of this study; (III) studies that had patient data in common; (IV) lack of adequate data prevents the extraction of diagnostic performance 2×2 data table research.
Study selection and data extraction
Following a duplicate check of the literature, both automated and manual, 2 investigators individually assessed the title and abstract of the article, eliminating irrelevant studies, and subsequently perused the complete text of the potentially suitable articles. They independently extracted data from eligible studies using pre-designed data tables, as indicated in Table S2. In the case of disagreement between the 2 investigators, a third investigator would engage in discussion with them until a consensus was reached.
Quality assessment
Using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (28), both investigators individually evaluated the potential for bias and the clinical relevance of every study, assessing the risk of bias and clinical applicability. The QUADAS-2 tool comprises 4 distinct sections, encompassing patient and lesion choice, index text, reference standard, and flow and timing. In the case of disagreement between the 2 investigators, a third investigator made the final decision after evaluating the reasons for both.
Statistical analysis
Statistical analysis was performed using Stata version 13.0 (StataCorp, College Station, TX, USA), Review Manager version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark), and Meta-DiSc 1.4 (Ramóny Cajal Hospital, Madrid, Spain). Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and 95% confidence interval (CI) were calculated using a bivariate mixed effects model. A summary receiver operating characteristic (sROC) curve was plotted and the area under the curve (AUC) was calculated. The closer the AUC was to 1, the more efficient the diagnosis. The evaluation of threshold effects was conducted using the Spearman correlation coefficient. The Q test and I2 index were employed to identify the heterogeneity of outcome measures across studies (if the Q test is less than or equal to 0.1 and I2 exceeds 50%, it may indicate significant heterogeneity). The heterogeneity was investigated by employing meta-regression analysis to examine the possible origin. Hypothesis tests were conducted using bilateral tests at a significance level of 0.05.
Results
Literature search
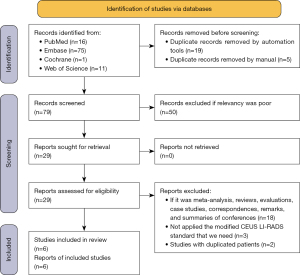
A total of 103 studies were retrieved, and 6 original studies were finally included. The research screening flow chart is shown in Figure 1.
Study characteristics
Table 1 displays the foundational data provided in the research. In the end, a grand total of 835 lesions were incorporated, comprising of 641 HCC, 98 OM, and 96 benign lesions. Table 2 displays the fundamental details of the encompassed lesions.
Table 1
| Author, year | Background | Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Centre | Study type | Study design | Years of enrollment | Image reviewer | No. of US systems | No. of patients | Average age (y) | Male, % |
Cirrhosis, % | ||
| Huang J, 2023 (17) | China | Single | Cohort | Prospective | 2021.06–2022.01 | Multiple | Multiple | 59 | 54 | 83.1 | 67.8§ | |
| Hwang JA, 2022 (18) | Korea | Multiple | Cohort | Retrospective | 2013.09–2020.06 | Multiple | Multiple | 123 | 61.5 | 76.7 | 41.5§ | |
| Liao W, 2023 (19) | China | Single | Cohort | Retrospective | 2020.01–2022.02 | Single | Multiple | 137 | 51 | 85.4 | 44.6§ | |
| Li L, 2022 (20) | China | Single | Cohort | Retrospective | 2020.03–2020.10 | Multiple | Single | 293 | 55 | 88.4 | 61.1§ | |
| Sugimoto K, 2020 (21) | Japan | Single | Cohort | Retrospective | 2017.03–2020.04 | Single | Single | 104 | 70.0 | 71.2 | 87.9‡ | |
| Takahashi H, 2022 (22) | Japan | Single | Cohort | Prospective | 2020.06–2021.07 | Single | Single | 102 | 71 | 62.7 | 79.4† | |
†, cirrhosis diagnosed by using ultrasound elastography; ‡, proportion of cirrhosis in the population with pathological findings; §, not reported method of diagnosis of cirrhosis. US, ultrasound.
Table 2
| Author, year | Lesions | Reference standards | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Average lesion size (mm) |
HCC, n (%) |
OM, n (%) |
Benign, n (%) |
Pathology, % | Interval from index test to pathology | Interval from index test to follow-up | ||
| Huang J, 2023 (17) | 62 | 3.5 (1.0–10.5)† | 55 (88.7)§¶ | 3 (4.8)§ | 4 (6.5)§¶ | 93.5 | NR | NR | |
| Hwang JA, 2022 (18) | 123 | 25 (10–130)† | 77 (62.6)§ | 15 (12.2)§ | 31 (25.2)§ǁ | 92.7 | ≤3 m | ≥2 y | |
| Liao W, 2023 (19) | 140 | 35.5 (23.8, 61.3)‡ | 119 (85.0)§ | 15 (10.71)§ | 6 (4.29)§ | 100 | ≤30 d | N/A | |
| Li L, 2022 (20) | 304 | 43 (6–158)† | 274 (90.1)§¶ | 14 (4.5)§ | 16 (5.4)§ǁ | 57.6 | ≤1 m | Benign ≥12 m | |
| Sugimoto K, 2020 (21) | 104 | 17.9 (13.1, 28.2)‡ | 64 (61.5)§ | 15 (14.4)§ | 25 (24.0)§ǁ | 87.5 | NR | ≥1 y | |
| Takahashi H, 2022 (22) | 102 | 25.5 (16.8, 44.3)‡ | 52 (51.0)§¶ | 36 (35.3)§ | 14 (13.7)§¶ǁ | 78.4 | NR | Some benign ≥6 m | |
†, median (range); ‡, median (interquartile range); §, pathological analysis; ¶, contrast-enhanced CT or MRI; ǁ, follow-up. HCC, hepatocellular carcinoma; OM, other non-HCC malignancies; d, days; m, months; y, years; NR, not reported; N/A, not applicable.
Quality assessment
As shown in Figure S1, a moderate level of bias was detected in the 6 studies’ overall risk. The risk of deviation mainly comes from reference standard and patient and lesion selection. A total of 3 studies exhibited potential bias in the reference standard by relying on CT/MRI for diagnosis without subsequent monitoring or follow-up. In terms of patient and lesion selection, 1 study excluded nodules that were markedly hyperechoic on gray-scale ultrasound. It was deemed to have a significant potential for bias. The other study did not specify if patients were included in consecutive order, which we deemed to have an uncertain level of risk. Regarding flow and timing, 3 studies failed to consider the time gap between CEUS examination and pathological findings, leading us to perceive the risk of bias as uncertain.
Diagnostic performance of LR5 for diagnosing HCC
In the modified CEUS LI-RADS, the LR-5 overall sensitivity for diagnosing HCC in high-risk individuals was 0.77 (95% CI: 0.70–0.82; I2=71.98%; P=0.00) (Figure 2A), and the overall specificity was 0.88 (95% CI: 0.83–0.92; I2=0.00; P=0.47) (Figure 2B). The diagnostic odds ratio (DOR) was 25.04 (95% CI: 15.04–41.67) (Figure 2C). Additionally, the AUC for modified CEUS LR5 was 0.91 (95% CI: 0.88–0.93) (Figure 3). The analysis of threshold effect indicated that there was no heterogeneity caused by threshold effect, as the Spearman correlation coefficient was 0.03 with a P value of 0.96.


Meta-regression analysis
The findings of the meta-regression analysis are summarized in Table 3. There was significant heterogeneity in sensitivity. Significant associations with study heterogeneity were found among the covariates examined, including study design, number of image reviewers, proportion of cirrhosis, proportion of OM cases, and type of reference standard (P≤0.05). The sensitivity of the retrospective analysis was greater than that of the prospective analysis (0.78 vs. 0.72, P=0.01). Multiple reviewers exhibited greater sensitivity compared to a single reviewer (0.82 vs. 0.69, P=0.01). The sensitivity of cirrhosis ≥50% and OM ≥10% was found to be lower compared to cirrhosis <50% (0.75 vs. 0.80, P=0.02) and studies with OM <10% (0.75 vs. 0.80, P=0.01). The study’s sensitivity, when relying solely on pathology, was inferior to that of the study that included both pathology and imaging follow-up (0.70 vs. 0.78, P=0.02).
Table 3
| Parameter | Sensitivity (95% CI) | P value |
|---|---|---|
| Study design | 0.01* | |
| Prospective (n=2) | 0.72 (0.60–0.85) | |
| Retrospective (n=4) | 0.78 (0.72–0.85) | |
| Image reviewer | 0.01* | |
| Multiple (n=3) | 0.82 (0.79–0.86) | |
| Single (n=3) | 0.69 (0.63–0.75) | |
| Cirrhosis% ≥50% | 0.02* | |
| Yes (n=4) | 0.75 (0.67–0.83) | |
| No (n=2) | 0.80 (0.70–0.90) | |
| OM% ≥10% | 0.01* | |
| Yes (n=4) | 0.75 (0.67–0.83) | |
| No (n=2) | 0.80 (0.7–0.94) | |
| Reference standard | 0.02* | |
| Pathology (n=1) | 0.70 (0.55–0.85) | |
| Mixed (n=5) | 0.78 (0.72–0.84) |
*, P<0.05. CEUS, contrast-enhanced ultrasound; LR5, Liver Imaging Reporting and Data System category 5; HCC, hepatocellular carcinoma; CI, confidence interval; OM, other non-HCC malignancies.
Discussion
Our study was conducted on the basis of 6 diagnostic tests to investigate the performance of Sonazoid-based modified CEUS LI-RADS LR-5 for the diagnosis of HCC. It was found that the modified CEUS LI-RADS LR-5 exhibited an overall sensitivity of 0.77, overall specificity of 0.88, DOR of 25.04, and AUC of 0.91 for the detection of HCC. There was a large heterogeneity in sensitivity (I2=71.98%) and no heterogeneity in specificity (I2=0.00) in our study. The heterogeneity was significantly associated with study design, number of image reviewers, proportion of liver cirrhosis, proportion of OM cases, and type of reference standard (P≤0.05).
In the recent meta-analyses on CEUS LI-RADS with blood pool agents, it was found that LR-5 had a sensitivity of 0.71, 0.69 and specificity of 0.93, 0.93 for diagnosing HCC (9,10). Our study utilized blood pools combined with Kupffer cells as contrast agents, resulting in an increase in sensitivity but a decrease in specificity compared to them. A recent meta-analysis using Sonazoid for intrahepatic HCC diagnostics reported combined sensitivity and specificity of 0.90 and 0.97, respectively (29). However, the included studies used different diagnostic criteria for HCC, which can affect its diagnostic performance and introduce potential bias. The results obtained in our study by uniformly using APHE combined with KP defects as a diagnostic criterion were different from those of the above studies. There is a possibility that the modified CEUS LI-RADS algorithm may decrease the specificity of diagnosing HCC. Decreased specificity results in more false-positive cases. False-positive diagnosis of HCC may lead to inappropriate or unneeded treatment of patients with OMs or benign lesions and LI-RADS is associated with the Organ Procurement and Transplantation Network (OPTN), so a high level of specificity is necessary for LI-RADS. Li et al. (20) showed that KP defects could be observed in 100% of OMs and 56.3% of benign lesions, and similarly, Kang et al. (30) found KP defects in 92% of malignant tumors and 33% of benign lesions when Sonazoid was used for CEUS. Hemangioma may show APHE and KP defects with Sonazoid, and can be misdiagnosed as HCC. Hemangiomas measuring less than 15 mm might display homogeneous hyperenhancement in the arterial phase, resembling the CEUS presentation of HCC (31). Research has indicated that some hepatic hemangiomas in CEUS using Sonazoid typically exhibit iso- or hypo-enhancement in the KP compared to the surrounding liver parenchyma (32). The primary factor contributing to the hypoenhancement of hemangiomas in the KP might be the manifestation of relatively reduced enhancement when Sonazoid is used, resulting from the enhanced peripheral liver parenchyma caused by Kupffer cell phagocytosis (32). This phenomenon is also present in MRI using hepatocyte-specific contrast agent and is known as the pseudo-washout effect (33,34). Therefore, we cannot rely solely on KP defects to distinguish benign and malignant tumors and HCC from OM, and we have to integrate gray-scale image features and portal phase information. As in the study by Hwang et al. (18), an additional diagnostic criterion was applied in order to avoid the reduced specificity of modified CEUS LI-RADS. That is, the downgrading of LR-5 nodules with indistinct borders and no hypoechoic halo on gray-scale ultrasound. The specificity of LR-5 for diagnosing HCC after downgrading increased from 0.84 to 0.91.
The findings of our research indicated that the modified CEUS LI-RADS exhibited a reasonable level of sensitivity in detecting HCC. The main diagnostic feature in KP defects replaces the mild and late (≥60 seconds) washout, distinguishing it from CEUS LI-RADS v2017. However, whether KP defects can improve the diagnostic sensitivity of HCC is controversial. A previous study reported that 13.4% of HCCs exhibited hypoenhancement solely in the KP, without showing it in the late portal venous phase (24). This could be due to the decrease in Kupffer cells that occurs before the decline in sinusoidal structure and portal blood flow within the tumor during HCC dedifferentiation. Several other studies observed the lesions at 1, 5, and 10 minutes after Sonazoid injection. They found that an increasing number of nodules exhibited hypoenhancement with longer observation time (15,22). The pathological examination of the lesions revealed that the majority of the lesions exhibiting hypoenhancement at 1 minute were HCCs with poor differentiation, whereas those not exhibiting hypoenhancement until 10 minutes were well-differentiated HCCs. Kupffer cells decrease with poor differentiation. In a separate investigation, the hypoenhancement of lesions was observed at different time intervals: 2, 5, and 10 minutes. Interestingly, the 5-minute interval demonstrated equal sensitivity and specificity compared to the 10-minute interval. However, when the 10-minute interval was employed, two false positive lesions were detected (35). Another study found that using a 6-minute cutoff for KP had high specificity and sensitivity, which did not significantly differ from using a 10-minute cutoff. The use of a 10-minute criterion did not lead to increased diagnosis of HCC (36). Therefore, although our research demonstrated reasonable sensitivity when considering KP defects as the primary criterion and surpassing SonoVue CEUS LI-RADS, further investigation is required to determine the cutoff time of KP and the role of KP defects in diagnosing HCC. When Sonazoid is applied for CEUS, the post-contrast image contains signals not only from the contrast agent, but also from tissue harmonic signals. Additionally, Sonazoid microbubbles require a moderate mechanical index (e.g., 0.2–0.3), which increases tissue harmonics and interferes with image observation, especially in KP images. If a hyperechoic lesion appears in grayscale ultrasound, it may interfere with the observation of portal phase washout and KP defects.
Joo et al. (34) investigated whether presenting hypointensity in the hepatobiliary phase (HBP) could serve as a substitute for washout in the portal phase when utilizing MRI with hepatocyte-specific contrast agent for diagnosing HCC. The study concluded that when combined with transitional phase (TP) or HBP, hypodensity increased diagnostic sensitivity but decreased specificity, similar to our findings using Sonazoid. They concluded that the portal-venous phase (PVP) should still be used as the main diagnostic criterion in order to ensure specificity. Unlike SonoVue, Sonazoid can be phagocytosed by Kupffer cells, and there is some overlap between the vascular phase and KP (7,14). Additionally, there is a transition period similar to that of the hepatocyte-specific MRI contrast agent TP. Therefore, it remains unclear to us whether and to what extent this imaging modality affects the washout pattern of the vascular phase of Sonazoid. KP defects could potentially serve as an ancillary feature for diagnosing HCC. Since there is a negative correlation between sensitivity and specificity, when it is not possible to harmonize the 2, it may be feasible to develop different algorithms to meet the therapeutic needs based on the varying requirements of these 2 factors in different regions.
Although the studies were carefully selected and assessed for methodological quality, there was heterogeneity in the statistical outcomes among the included studies. Inter-study heterogeneity may arise from random factors, errors in analytical methods, differences in patient selection and clinical settings, disease severity, details of indicators and reference tests, as well as interobserver variability (37,38). In our analysis, the 4 retrospective studies had higher sensitivity than the 2 prospective studies. Ensuring the authenticity and completeness of retrospective study records is challenging, leading to a low level of evidence and a potentially high risk of bias (37). Images reviewed by multiple individuals exhibited higher sensitivity compared to those reviewed by a single individual. When multiple people review the image and reach an agreement through discussion or arbitration, a more comprehensive analysis of the image can be conducted, thus avoiding the omission of important information that may occur when reviewed by a single person. Studies with a greater percentage of cirrhosis patients showed decreased sensitivity in comparison to those with a lower percentage. Severe cirrhosis frequently presents with a rough texture of the liver tissue, showing widespread shrinkage and a higher prevalence of Kupffer cell depletion and/or compromised functionality. Multiple nodules at different stages of HCC development, such as dysplastic nodules (DN), early HCC, and so on, may be present in the context of cirrhosis. Therefore, the identification of benign and malignant nodules in the setting of cirrhosis may be a challenge (14). Studies with high proportions of OM have shown relatively lower sensitivity than those with low proportions. The heterogeneity of studies when using CEUS LI-RADS can be attributed to the variation in the performance of the index test, which is influenced by the prevalence of the disease (39). A total of 5 studies, which utilized either pathology or imaging diagnosis as a reference standard, demonstrated higher sensitivity compared to a single study that solely relied on pathology. Studies that utilize an alternative imaging finding as a reference standard without follow-up may encounter potential biases related to reference standard and inclusion (40).
Our meta-analysis had several limitations. The main methodological limitation is the scarcity of original studies on modified CEUS LI-RADS, particularly prospective and head-to-head studies. The estimation of the diagnostic performance of modified CEUS LI-RADS and the identification of sources of heterogeneity could be impacted by this. Additionally, it can enhance the impact of individual research and skew the findings of the present study (41). Further, more comprehensive original research is anticipated to be conducted in order to investigate the applicability of CEUS LIRADS version for Sonazoid contrast agent. Secondly, Sonazoid contrast agent had been approved for use in a few Asian countries, and the included studies were all from these countries, but there are regional variations in the etiology of HCC (1). In Asian countries, there is a higher prevalence of chronic infection caused by the hepatitis B virus or hepatitis C virus, whether or not cirrhosis is present. Meanwhile, the rise in prevalence in European countries in recent years can be attributed to the increase in overweight individuals and cases of diabetes. Therefore, these studies may not have been able to cover a larger population and the results may be biased. Furthermore, the limited quantity of OM lesions and non-malignant lesions incorporated in this meta-analysis might have influenced the evaluation of specificity. In addition, because of inadequate data reporting, we refrained from conducting additional subgroup analyses considering factors such as lesion size and the cause of liver disease.
Conclusions
The modified CEUS LI-RADS LR-5 categorization demonstrates a reasonable level of sensitivity but an insufficient level of specificity when diagnosing HCC. KP defects cannot be used as a primary feature in the diagnosis of HCC by CEUS LI-RADS, although perhaps as an ancillary feature.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1184/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1184/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583-705. [Crossref] [PubMed]
- Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021;10:181-223. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Bureau of Medical Administration, National Health Commission of the People's Republic of China. Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition). Chinese Journal of Digestive Surgery 2022;21:143-68.
- Dietrich CF, Nolsoe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver - Update 2020 - WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med 2020;41:562-85. [Crossref] [PubMed]
- American College of Radiology. CEUS LI-RADS v2017 core. Available online: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/CEUS-LI-RADS-2017-Core.pdf. Accessed 14 July 2020.
- Jin H, Cai Y, Zhang M, Huang L, Bao W, Hu Q, Chen X, Zhou L, Ling W. LI-RADS LR-5 on contrast-enhanced ultrasonography has satisfactory diagnostic specificity for hepatocellular carcinoma: a systematic review and meta-analysis. Quant Imaging Med Surg 2023;13:957-69. [Crossref] [PubMed]
- Li L, Hu Y, Han J, Li Q, Peng C, Zhou J. Clinical Application of Liver Imaging Reporting and Data System for Characterizing Liver Neoplasms: A Meta-Analysis. Diagnostics (Basel) 2021.
- Sontum PC. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med Biol 2008;34:824-33. [Crossref] [PubMed]
- Watanabe R, Matsumura M, Chen CJ, Kaneda Y, Ishihara M, Fujimaki M. Gray-scale liver enhancement with Sonazoid (NC100100), a novel ultrasound contrast agent; detection of hepatic tumors in a rabbit model. Biol Pharm Bull 2003;26:1272-7. [Crossref] [PubMed]
- Shunichi S, Hiroko I, Fuminori M, Waki H. Definition of contrast enhancement phases of the liver using a perfluoro-based microbubble agent, perflubutane microbubbles. Ultrasound Med Biol 2009;35:1819-27. [Crossref] [PubMed]
- Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, et al. The AFSUMB Consensus Statements and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound using Sonazoid. Ultrasonography 2020;39:191-220. [Crossref] [PubMed]
- Takahashi M, Maruyama H, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound with perflubutane microbubble agent: evaluation of differentiation of hepatocellular carcinoma. AJR Am J Roentgenol 2011;196:W123-31. [Crossref] [PubMed]
- Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018;289:816-30. [Crossref] [PubMed]
- Huang J, Gao L, Li J, Yang R, Jiang Z, Liao M, Luo Y, Lu Q. Head-to-head comparison of Sonazoid and SonoVue in the diagnosis of hepatocellular carcinoma for patients at high risk. Front Oncol 2023;13:1140277. [Crossref] [PubMed]
- Hwang JA, Jeong WK, Kang HJ, Lee ES, Park HJ, Lee JM. Perfluorobutane-enhanced ultrasonography with a Kupffer phase: improved diagnostic sensitivity for hepatocellular carcinoma. Eur Radiol 2022;32:8507-17. [Crossref] [PubMed]
- Liao W, Que Q, Wen R, Lin P, Chen Y, Pang J, Guo D, Wen D, Yang H, He Y. Comparison of the Feasibility and Diagnostic Performance of ACR CEUS LI-RADS and a Modified CEUS LI-RADS for HCC in Examinations Using Sonazoid. J Ultrasound Med 2023;42:2501-11. [Crossref] [PubMed]
- Li L, Zheng W, Wang J, Han J, Guo Z, Hu Y, Li X, Zhou J. Contrast-Enhanced Ultrasound Using Perfluorobutane: Impact of Proposed Modified LI-RADS Criteria on Hepatocellular Carcinoma Detection. AJR Am J Roentgenol 2022;219:434-43. [Crossref] [PubMed]
- Sugimoto K, Kakegawa T, Takahashi H, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Kasai Y, Itoi T. Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid. Diagnostics (Basel) 2020;10:828. [Crossref] [PubMed]
- Takahashi H, Sugimoto K, Kamiyama N, Sakamaki K, Kakegawa T, Wada T, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Itoi T. Noninvasive Diagnosis of Hepatocellular Carcinoma on Sonazoid-Enhanced US: Value of the Kupffer Phase. Diagnostics (Basel) 2022;12:141. [Crossref] [PubMed]
- Sugimoto K, Saito K, Shirota N, Kamiyama N, Sakamaki K, Takahashi H, Wada T, Kakegawa T, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Itoi T. Comparison of modified CEUS LI-RADS with sonazoid and CT/MRI LI-RADS for diagnosis of hepatocellular carcinoma. Hepatol Res 2022;52:730-8. [Crossref] [PubMed]
- Hwang JA, Jeong WK, Min JH, Kim YY, Heo NH, Lim HK. Sonazoid-enhanced ultrasonography: comparison with CT/MRI Liver Imaging Reporting and Data System in patients with suspected hepatocellular carcinoma. Ultrasonography 2021;40:486-98. [Crossref] [PubMed]
- van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, Tang A, Singal AG, Costa AF, Fowler K, McInnes MDF. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology 2019;156:976-86. [Crossref] [PubMed]
- Shin J, Lee S, Bae H, Chung YE, Choi JY, Huh YM, Park MS. Contrast-enhanced ultrasound liver imaging reporting and data system for diagnosing hepatocellular carcinoma: A meta-analysis. Liver Int 2020;40:2345-52. [Crossref] [PubMed]
- Wang H, Cao J, Fan H, Huang J, Zhang H, Ling W. Compared with CT/MRI LI-RADS, whether CEUS LI-RADS is worth popularizing in diagnosis of hepatocellular carcinoma?-a direct head-to-head meta-analysis. Quant Imaging Med Surg 2023;13:4919-32. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Yang Y, Liu C, Yan J, Liu K. Perfluorobutane contrast-enhanced ultrasonography for the diagnosis of HCC: a systematic review and meta-analysis. Abdom Radiol (NY) 2021;46:4619-28. [Crossref] [PubMed]
- Kang HJ, Lee JM, Yoon JH, Lee K, Kim H, Han JK. Contrast-enhanced US with Sulfur Hexafluoride and Perfluorobutane for the Diagnosis of Hepatocellular Carcinoma in Individuals with High Risk. Radiology 2020;297:108-16. [Crossref] [PubMed]
- Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology 2007;45:1139-45. [Crossref] [PubMed]
- Sugimoto K, Moriyasu F, Saito K, Yoshiara H, Imai Y. Kupffer-phase findings of hepatic hemangiomas in contrast-enhanced ultrasound with sonazoid. Ultrasound Med Biol 2014;40:1089-95. [Crossref] [PubMed]
- Doo KW, Lee CH, Choi JW, Lee J, Kim KA, Park CM. "Pseudo washout" sign in high-flow hepatic hemangioma on gadoxetic acid contrast-enhanced MRI mimicking hypervascular tumor. AJR Am J Roentgenol 2009;193:W490-6. [Crossref] [PubMed]
- Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol 2015;25:2859-68. [Crossref] [PubMed]
- Kang HJ, Lee JM, Yoon JH, Yoo J, Choi Y, Joo I, Han JK. Sonazoid™ versus SonoVue(®) for Diagnosing Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound in At-Risk Individuals: A Prospective, Single-Center, Intraindividual, Noninferiority Study. Korean J Radiol 2022;23:1067-77. [Crossref] [PubMed]
- Kang HJ, Kim JH, Yoo J, Han JK. Diagnostic criteria of perfluorobutane-enhanced ultrasonography for diagnosing hepatocellular carcinoma in high-risk individuals: how is late washout determined? Ultrasonography 2022;41:530-42. [Crossref] [PubMed]
- Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part I. General Guidance and Tips. Korean J Radiol 2015;16:1175-87. [Crossref] [PubMed]
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. [Crossref] [PubMed]
- Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ 2013;185:E537-44. [Crossref] [PubMed]
- Sica GT. Bias in research studies. Radiology 2006;238:780-9. [Crossref] [PubMed]
- Cronin P, Kelly AM, Altaee D, Foerster B, Petrou M, Dwamena BA. How to Perform a Systematic Review and Meta-analysis of Diagnostic Imaging Studies. Acad Radiol 2018;25:573-93. [Crossref] [PubMed]


