
Determination of axillary lymph node status by fine-needle aspiration: preoperative axillary staging vs. postoperative surveillance for breast cancers
Introduction
Breast cancer is the most common cancer among women worldwide and in South Korea (1,2). For the preoperative staging of breast cancer, regional lymph node (LN) status is one of the strongest predictors of long-term prognosis and is important for planning treatment (3,4). If axillary node (AN) metastasis is confirmed by fine-needle aspiration (FNA) during staging work-up, neoadjuvant chemotherapy (NAC) or upfront AN dissection can be performed (5,6). In contrast, if there is no suspicious AN on preoperative ultrasound (US), sentinel LN (SLN) surgery is performed to select patients for whom axillary dissection can be omitted (7). Therefore, preoperative axillary US and AN-FNA can influence axillary surgical plans and the choice of NAC. When there are ANs showing suspicious features on US, surgeons or radiologists want to verify the pathologic results in order to avoid false-positive results and reduce fruitless AN dissection. Although AN-FNAs may help triage equivocal cases to whether NAC or conventional surgery, indiscriminate FNAs may be more harmful. In order to work smarter, we should reduce time-consuming and unnecessary FNAs that often lead to negative results through feedback by outcome monitoring. The outcome monitoring of assessment of axillary status on US by FNA can be a guideline for daily practice to predict a lymph node metastasis.
Meanwhile, the axillary US can be used for postoperative imaging surveillance of women with a personal history of breast cancer (PHBC). In these women, axillary scanning is optional in the ACRIN 6666 protocol (8). However, in our previous study, among the occult recurrences during locoregional US for the breast and axillary areas in women with PHBC, 33% of recurrences were present in the axillary area (9). In countries where whole-breast US has been used as a supplemental screening modality, axillary scanning can be easily adopted in the routine protocol and the detected abnormalities need to be characterized.
Similar to breast US, the axillary US also has a tendency for subjective reporting, making communication difficult. Although there are some suggested US features of malignant LNs, such as cortical abnormalities, including focal or diffuse thickening >3 mm, presence of focal bulges, and peripheral vascularization on color Doppler (10-15), only a few studies have reported the malignancy likelihood of AN on US according to an imaging-based grading system (15,16). If a reporting system similar to the Breast Imaging Reporting and Data System (BI-RADS) (17) is available for axillary US and the system can suggest benchmarks for the malignancy likelihood, it will help ensure the quality of axillary US practices. The validation should be made by means of US-guided FNA or surgical biopsy. US-guided FNA can be easily applied for suspicious ANs and it has been widely accepted in diagnostic examinations. However, the diagnostic accuracy can be different according to the clinical setting whether in diagnostic during staging work-up or in surveillance setting in women with PHBC. There is a paucity of studies on the diagnostic performance of AN-FNA in breast cancer surveillance groups, and the study population is relatively small (18). Through the analysis of the malignancy likelihood of AN-FNA in a larger study population, an ideal guideline can be suggested. Following the SOUND trial, which was conducted between 2012 and 2017 in Europe and published results supporting the omission of sentinel node biopsy (19), our study was conducted to evaluate the diagnostic performance of practices that performed US-guided AN-FNA based on US features and implement quality control.
In this study, we investigated the outcome of US-guided AN-FNA performed in breast cancer patients according to the axillary node-reporting and data system (AN-RADS) category, modified from BI-RADS, which is very familiar to the breast radiologists, and compared the results between two different clinical settings: staging and surveillance groups. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1452/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Samsung Medical Center and individual consent for this retrospective analysis was waived. We consecutively enrolled patients who underwent US-guided AN-FNA from 1 January 2017 to 31 December 2017 in Samsung Medical Center in Seoul. Patients whose primary cancer was not breast cancer were excluded. Among 769 US-guided AN-FNA procedures, two patients were excluded and total 767 procedure were performed for ANs that were suspicious or requested for cytology in 765 consecutive patients specified with breast cancers in our institution. In two patients, repeated FNA was performed on the same axilla on two different days. The reason for axillary US was the preoperative staging of current breast cancers in 654 cases and postoperative surveillance for women with PHBC in 113 cases (Figure 1).
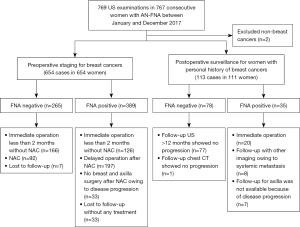
US examination and pathologic analyses
US was performed using various machines with a high-resolution (4–15 MHz) linear array transducer (iU22, Philips Medical Systems, Bothell, WA, USA; RS80A, Samsung Medison Co. Ltd., Seoul, Korea; Supersonic Imagine, Aix en Provence, France). One of the 15 board-certified radiologists performed the US and US-guided procedures. All practitioners were specialized in breast imaging (nine staff radiologists with 7–20 years of experience and six fellow radiologists with 3–27 months of experience). US-guided AN-FNA was performed using a 23-gauge needle with a 2-cc syringe. Cytological findings from AN-FNA have been reported as benign or malignant. Surgical pathology results were available in less than 2 months in 312 cases (166 FNA-negative and 126 FNA-positive cases in the staging group, and 20 FNA-positive cases in the surveillance group) and follow-up imaging with US or computed tomography (CT) for more than a year was available in 86 cases (78 FNA negative and 8 FNA positive cases in the surveillance group) (Figure 1). Patients with false-negative FNA findings were explored based on the final surgical pathology or imaging follow-up for >1 year.
Axillary node reporting and data system (AN-RADS)
For each case, the radiologists prospectively assigned a five-scale category to the most suspicious ANs based on US morphology according to the level of suspicion at the time of the procedure. Hereafter, we will refer to this system as the AN-RADS category based on the concept of 7-scale BI-RADS categories familiar to breast radiologists and clinicians to improve communication. Of the 7-scale categories, we included only 5-scale categorization system which could be the subjects of diagnostic FNA, as follows: 3, high-probability benign; 4A, low suspicion; 4B, moderate suspicion; 4C, high-suspicion; and 5, high-probability malignancy.
If the representative LN at the lower axillary border (presumed to be a sentinel node) had an echogenic hilum and an oval shape with a cortex ≤3 mm, morphologically similar to the remaining axillary LNs in the ipsilateral level I axilla, we considered it as category 3 (high-probability benign). When the LN showed diffuse cortical thickening more than 3 mm with an equivocal shape, category 4A (low-suspicion) was assigned. When the interpreters thought the LN was quite suspicious, showing combination of suspicious US findings, including eccentric cortical thickening (>3 mm), round LNs with a blurry or partially compressed fatty hilum, partial or complete loss of the fatty hilum, the LNs were categorized as 4B (moderate-suspicion) or 4C (high-suspicion) according to the degree of suspicion. Category 5 (high-probability malignant) was defined as an LN completely replaced with an irregular mass, an LN showing extracapsular extension and microcalcifications (Figure 2).
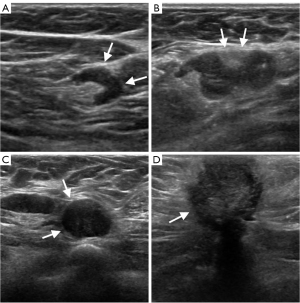
To reduce inter-reader variability, cases with radiologic pathological discrepancies were reviewed through a weekly departmental supervisory meeting, and the interpreters who provided inappropriate categories before FNA received corrective feedback.
Data analysis and statistical methods
Malignancy rates for each AN-RADS category in the staging and surveillance groups were calculated. The chi-square test, with or without Bonferroni correction, or Fisher’s exact test was used to compare the distribution of the assigned category and malignancy rates between the staging and surveillance groups for each category. The Cochran-Armitage trend test was performed to assess the relationship between the increase in AN-RADS category and malignancy rate. Logistic regression analysis using Firth’s penalized likelihood approach was used to compare the relationship between the malignancy rate and the assigned categories between the two groups. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A two-sided P value of <0.05 was considered statistically significant.
Results
Among 769 US-guided AN-FNA, this study included 767 procedures in 765 patients. Only two patient were excluded due to a primary cancer other than breast cancer. Two of the 767 patients had two US-guided AN-FNAs. The breast cancer type of study population and distribution of the AN-RADS categories are summarized in Table 1. The distribution of ductal and lobular types, excluding the others type, was not statistically significantly different in the two groups. A category 4A (low-suspicion) was most commonly given in both the staging and surveillance groups (48.0% and 63.7%, respectively) and was given more commonly in the surveillance group than in the staging group (P=0.002). The distribution of categories differed between the two groups (P=0.015).
Table 1
| Variables | Staging | Surveillance | P value |
|---|---|---|---|
| Age (years), median [1st, 3rd] | 49 [43, 57] | 53 [47, 61] | 0.0001* |
| Breast cancer type, n (%) | 0.001** | ||
| Ductal (IDC and DCIS) | 613 (93.7) | 100 (88.5) | |
| ILC | 29 (4.4) | 4 (3.5) | |
| Others† | 12 (1.8) | 9 (8.0) | |
| AN-RADS category, n (%) | 0.015** | ||
| 3 (high-probability benign) | 18 (2.8) | 4 (3.5) | 0.551 |
| 4A (low-suspicion) | 314 (48.0) | 72 (63.7) | 0.002 |
| 4B (moderate-suspicion) | 137 (20.9) | 15 (13.3) | 0.059 |
| 4C (high-suspicion) | 122 (18.7) | 18 (15.9) | 0.489 |
| 5 (high-probability malignant) | 63 (9.6) | 4 (3.5) | 0.031 |
| Total | 654 (100) | 113 (100) |
†, includes metaplastic carcinoma, mucinous carcinoma, solid papillary carcinoma, invasive micropapillary carcinoma, mixed mucinous and micropapillary carcinoma, tubular carcinoma, invasive carcinoma with pleomorphic large cells, signet ring cell carcinoma; *, Wilcoxon rank sum test; **, Chi-squared test. AN-RADS, axillary nodal reporting and data system; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma.
Of the 767 ANs, 424 (55.3%) were found to be malignant. The malignancy rate was significantly different between the staging and surveillance groups: 59.5% (389/654) in the staging group and 31.0% (35/113) in the surveillance group (P<0.001) (Table 2). The malignancy rates in the staging and surveillance groups according to AN-RADS category were 5.6% vs. 0.0% for category 3 (high-probability benign), 36.0% vs. 9.7% for category 4A (low-suspicion), 77.4% vs. 53.3% for category 4B (moderate-suspicion), 87.7% vs. 88.9% for category 4C (high-suspicion), and 98.4% vs. 100% for category 5 (high-probability malignant), respectively. The malignancy rate was significantly different between the staging and surveillance groups for category 4A (P=0.0001). An increase in the malignancy rate along with a higher suspicion category was noted (P<0.001), and this trend was not significantly different between the two groups (P=0.41).
Table 2
| AN-RADS category | Staging (n=654), n (%) [95% CI] |
Surveillance (n=113), n (%) [95% CI] |
P value |
|---|---|---|---|
| 3 (high-probability benign) | 1 (5.6) [0.14–27.29] | 0 (0.0) | >0.99 |
| 4A (low-suspicion) | 113 (36.0) [30.7–41.6] | 7 (9.7) [4.0–19.0] | 0.0001 |
| 4B (moderate-suspicion) | 106 (77.4) [69.5–84.1] | 8 (53.3) [26.6–78.7] | 0.29 |
| 4C (high-suspicion) | 107 (87.7) [80.5–93.0] | 16 (88.9) [65.3–98.6] | >0.99 |
| 5 (high-probability malignant) | 62 (98.4) [91.5–100.0] | 4 (100.0) [39.8–100.0] | >0.99 |
| All | 389 (59.5) | 35 (31.0) | <0.001 |
AN-FNA, axillary nodal fine-needle aspiration; AN-RADS, axillary nodal reporting and data system; CI, confidence interval.
In the staging group (n=654), 292 cases were available for immediate surgery within 2 months. Among these false-negative cases of AN-FNA occurred in 48 of 166 cases with negative FNA results (28.9%), and all were in the staging group. Of these 48, 39 (81.3%) were found to have one to two LN metastases, and 9 (18.8%) were found to have heavy-burden metastases of up to three or more LNs. Heavy-burden metastasis with false-negative results was assessed in category 4A (low suspicion) in six patients and category 4B (moderate suspicion) in three patients. Surgically confirmed size of metastatic deposits in the false negative ANs was 0.025–2.0 cm (mean: 0.58 cm, median: 0.4 cm).
In the surveillance group, there were no proven false-negative or false-positive results. All 78 FNA-negative patients in the surveillance group had imaging follow-up results (US in 77 patients between 12 and 21 months and chest CT in one patient), the LNs showed no interval change or decrease in size or conspicuity, and the degree of suspicion was lowered. Two patients underwent repeat FNA of the same or different LNs, which revealed negative results. Among the 35 FNA-positive cases in the surveillance group, 20 underwent surgery for metastatic LNs, and the others were regarded as true positives due to concomitant systemic metastasis or further progression of metastasis on follow-up imaging or were lost to follow-up due to self-withdrawal of further treatment.
Discussion
We investigated the malignancy rate of AN-FNA according to the AN-RADS category in breast cancer patients and evaluated the difference in the malignancy rate in two different clinical settings (staging and surveillance). The rate was significantly higher in the staging group (59.5%) than in the surveillance group (31.0%) (P<0.001). The malignancy rates of each category were as follows: 5.6%, 36.0%, 77.4%, 87.7%, and 98.4% vs. 0.0%, 9.7%, 53.3%, 88.9%, and 100%, respectively, in the staging and surveillance groups. The rate showed an increasing tendency with increasing categories, and the outcome according to the AN-RADS category in the surveillance group was concordant with the BI-RADS guidelines for breast lesions (0–2%, >2–10%, >10–50%, >50–95%, and >95–100% in categories 3, 4A, 4 B, 4C, and 5, respectively). Although a higher malignancy rate was observed in the staging group than in the surveillance group in the 3, 4A, and 4B categories, also quite different from the BI-RADS guidelines, the difference was only statistically significant in category 4A (P=0.0001). We suggest the reason for the higher malignancy rate of ANs per AN-RADS category in staging group than in surveillance group is that the prevalence of metastasis in the staging group who have not yet been treated is 59.5%, which is higher than the prevalence of metastasis in the surveillance group (31.0%) (P<0.001).
Previous study dealing with US FNA including normal-appearing LN groups showed 11% sensitivity in normal-appearing LNs, 44% sensitivity in indeterminate LNs, and 93% sensitivity in suspicious LNs for patients who were candidates for SLN surgery (15). The result of the indeterminate LNs in the previous study was between the values of 4A and 4B in staging group of our study. This difference is probably due to the different target populations; the previous study only included patients who were candidates for sentinel LN surgery.
In our study, the malignancy rate of ANs in women with preoperative breast cancer (59.5%) was consistent with the results of a previous report (20,21). The higher malignancy rate of ANs in the staging group indicates that abnormal ANs discovered before surgery for breast cancer have a higher potential for metastasis than abnormal ANs found in the surveillance follow-up US, even with the same abnormal US morphologic findings. According to the benchmarks for diagnostic and screening mammography in the follow-up and outcome monitoring chapter of the ACR BI-RADS atlas, the performance differed in the screening settings, diagnostic settings following abnormal screening, and diagnostic settings with palpable lumps. The cancer detection rates per 1,000 examinations were 2.5, >20, and >40 in each clinical setting (17). This is similar to the results of our study, in which the malignancy rate of FNA was 31.0% in the surveillance screening and 59.5% in the diagnostic staging.
There were 48 false-negative cases of US-guided AN-FNA, and all cases were in the staging group; these were revealed as metastasis following surgery. Rarely, there are cases showing unexpectedly high nodal staging with negative FNA results. However, this false-negative rate was acceptable because > 80% of missed detections were one or two LN metastases, which is a low-burden nodal metastasis that is usually managed with sentinel LN biopsy during the operation.
In previous studies, the false-negative rate of preoperative US-guided axillary FNA is associated with smaller lymph node size (<1.2 cm), smaller cortical thickness (<3.5 mm), and a lower percentage of lymph node replacement by carcinoma (22). The main reason of false negative results might be the failure to identify small metastases in LNs with normal appearance or the identification failure of LN itself. As the axillary region has no distinct anatomical landmarks, it is difficult to find all the small axillary LNs. The second reason might be the selection error; the targeted LN during US-guided FNA could be different from the histologically metastatic LNs. It can happen when the multiple half-suspicious LNs were found during US. The last possible reason could be technical problems in FNA and the inherent limitations of cytological interpretation. In this context, accurate LN selection is important to reduce the false-negative rate, and contrast-enhanced ultrasound (CEUS) or elastography added to greyscale US may help to improve diagnostic performance (23-26).
The possible reason for the higher false negative rate in the staging group than in the surveillance group is that since the gold standard for the staging group is surgery and the surveillance group is US follow-up, it is possible that micrometastasis was not clinically detectable in the surveillance group. Also, since the staging group is a group of patients before treatment for breast cancer, the underlying malignancy prevalence was higher than in the surveillance group, which may have contributed to the higher false negative rate.
This study has several limitations. First, it was a retrospective, single-institution study. Thus, there might have been a selection bias in the inclusion of study patients. However, this study included a consecutive series of patients. Second, only lesions that had been diagnosed using AN-FNA were included in this study. Other benign-looking ANs not tested by AN-FNA were also excluded. Therefore, the results of our study do not reflect the sensitivity or specificity of the total AN US. However, we think our results showed the outcome of AN-FNA for LNs above a minimal suspicion level. Third, there could be inter-reader variability in the suspicion category assessment and differences in procedural skillfulness between radiologists. However, all radiologists included in this study specialized in breast imaging for 7–20 years or were on fellowship training. In addition, to reduce inter-reader variability, cases with radiologic and pathological discrepancies were reviewed at weekly breast department supervisory meetings. Fourth, AN-FNA rather than core needle biopsy (CNB) was performed to assess AN status in this study. Previous studies have reported that AN-CNB has better diagnostic performance than AN-FNA (27-29), although the differences were not significant. In a meta-analysis of the diagnostic accuracy of FNA cytology and CNB in the assessment of ANs in breast cancer, Pyo et al. reported that both FNA and CNB are useful in preoperative assessments of ANs in patients with breast cancer (30). Furthermore, AN-CNB has limitations in the use of narrow spaces and is in close proximity to large vessels, and the use of a small LN could be a diagnostic challenge. Fifth, reactive LN secondary to breast biopsy may alter the morphology. Most AN-FNA was performed after breast biopsy in the staging group, which may have affected the LN morphology. Finally, since the FNA results in the surveillance group were not confirmed by surgical pathology, but were considered true negatives if they did not progress at 1 year or more of follow-up, there is the possibility that some of the cases containing non-viable microscopic metastatic foci were mistaken for true negatives because they did not progress to clinically detectable metastasis. The recent result of SOUND trial addressed that the patients with small (<2 cm) breast cancers and sonographically normal appearing lymph nodes with no axillary surgery and with appropriate chemo-radiation therapy showed very low LN recurrence rate in the axilla (0.4% at 5 years) (19). There is a chance that the metastasis not removed in surgery do not develop as clinically detectable metastasis during surveillance US.
Conclusions
In conclusion, the malignancy rate of ANs per AN-RADS category was generally higher in the staging group than in the surveillance group in the patients specified with breast cancers, probably because of differences in the risk of malignancy. The difference was quite significant in category 4A low-suspicion LNs (36.0% vs. 9.7%). We suggest that examiners should expect a higher malignancy possibility of AN with suspicious findings noted on the preoperative US. We also demonstrated the outcomes of the AN-RADS based on US morphology with an assessment of the malignancy rate. These results may help design a generalized AN reporting system that can provide an objective range of suspicion levels of malignancy and may help communication between radiologists and clinicians and research on axillary US in patients with breast cancer. Future research is needed to suggest and validate the systematic classification of suspicion levels according to imaging findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1452/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1452/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Samsung Medical Center and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Jung KW, Won YJ, Hong S, Kong HJ, Lee ES. Prediction of Cancer Incidence and Mortality in Korea, 2020. Cancer Res Treat 2020;52:351-8. [Crossref] [PubMed]
- Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v8-30. [Crossref] [PubMed]
- Banerjee M, George J, Song EY, Roy A, Hryniuk W. Tree-based model for breast cancer prognostication. J Clin Oncol 2004;22:2567-75. [Crossref] [PubMed]
- Aigner J, Schneeweiss A, Sohn C, Marmé F. The role of neoadjuvant chemotherapy in the management of primary breast cancer. Minerva Ginecol 2011;63:261-74.
- Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. [Crossref] [PubMed]
- Kim GR, Choi JS, Han BK, Lee JE, Nam SJ, Ko EY, Ko ES, Lee SK. Preoperative Axillary US in Early-Stage Breast Cancer: Potential to Prevent Unnecessary Axillary Lymph Node Dissection. Radiology 2018;288:55-63. [Crossref] [PubMed]
- Shin SU, Chang JM, Park J, Lee HB, Han W, Moon WK. The Usefulness of Ultrasound Surveillance for Axillary Recurrence in Women With Personal History of Breast Cancer. J Breast Cancer 2022;25:25-36. [Crossref] [PubMed]
- Shin JH, Han BK, Choe YH, Nam SJ, Park W, Im YH. Ultrasonographic detection of occult cancer in patients after surgical therapy for breast cancer. J Ultrasound Med 2005;24:643-9. [Crossref] [PubMed]
- Maxwell F, de Margerie Mellon C, Bricout M, Cauderlier E, Chapelier M, Albiter M, Bourrier P, Espié M, de Kerviler E, de Bazelaire C. Diagnostic strategy for the assessment of axillary lymph node status in breast cancer. Diagn Interv Imaging 2015;96:1089-101. [Crossref] [PubMed]
- Cho N, Moon WK, Han W, Park IA, Cho J, Noh DY. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol 2009;193:1731-7. [Crossref] [PubMed]
- Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol 2006;186:1342-8. [Crossref] [PubMed]
- Esen G, Gurses B, Yilmaz MH, Ilvan S, Ulus S, Celik V, Farahmand M, Calay OO. Gray scale and power Doppler US in the preoperative evaluation of axillary metastases in breast cancer patients with no palpable lymph nodes. Eur Radiol 2005;15:1215-23. [Crossref] [PubMed]
- Lee B, Lim AK, Krell J, Satchithananda K, Coombes RC, Lewis JS, Stebbing J. The efficacy of axillary ultrasound in the detection of nodal metastasis in breast cancer. AJR Am J Roentgenol 2013;200:W314-20. [Crossref] [PubMed]
- Mainiero MB, Cinelli CM, Koelliker SL, Graves TA, Chung MA. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol 2010;195:1261-7. [Crossref] [PubMed]
- De Coninck C, Noël JC, Boutemy R, Simon P. Preoperative axillary lymph node staging by ultrasound-guided cytology using a four-level sonographic score. BMC Med Imaging 2016;16:13. [Crossref] [PubMed]
- D’Orsi CJ. ACR BI-RADSR Atlas : mammo- graphy, breast ultrasound, breast MR imaging. 4th ed. Reston, VA: American College of Radiology, 2003.
- Hammon M, Dankerl P, Janka R, Wachter DL, Hartmann A, Schulz-Wendtland R, Uder M, Wenkel E. Fine needle aspiration cytology of lymph nodes in breast cancer follow-up is a feasible alternative to watchful waiting and to histology. BMC Womens Health 2015;15:114. [Crossref] [PubMed]
- Gentilini OD, Botteri E, Sangalli C, Galimberti V, Porpiglia M, Agresti R, et al. Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. JAMA Oncol 2023;9:1557-64. [Crossref] [PubMed]
- Nori J, Vanzi E, Bazzocchi M, Bufalini FN, Distante V, Branconi F, Susini T. Role of axillary ultrasound examination in the selection of breast cancer patients for sentinel node biopsy. Am J Surg 2007;193:16-20. [Crossref] [PubMed]
- Samiei S, van Nijnatten TJA, van Beek HC, Polak MPJ, Maaskant-Braat AJG, Heuts EM, van Kuijk SMJ, Schipper RJ, Lobbes MBI, Smidt ML. Diagnostic performance of axillary ultrasound and standard breast MRI for differentiation between limited and advanced axillary nodal disease in clinically node-positive breast cancer patients. Sci Rep 2019;9:17476. [Crossref] [PubMed]
- Ewing DE, Layfield LJ, Joshi CL, Travis MD. Determinants of False-Negative Fine-Needle Aspirates of Axillary Lymph Nodes in Women with Breast Cancer: Lymph Node Size, Cortical Thickness and Hilar Fat Retention. Acta Cytol 2015;59:311-4. [Crossref] [PubMed]
- Du LW, Liu HL, Gong HY, Ling LJ, Wang S, Li CY, Zong M. Adding contrast-enhanced ultrasound markers to conventional axillary ultrasound improves specificity for predicting axillary lymph node metastasis in patients with breast cancer. Br J Radiol 2021;94:20200874. [Crossref] [PubMed]
- Steppan I, Reimer D, Müller-Holzner E, Marth C, Aigner F, Frauscher F, Frede T, Zeimet AG. Breast cancer in women: evaluation of benign and malignant axillary lymph nodes with contrast-enhanced ultrasound. Ultraschall Med 2010;31:63-7. [Crossref] [PubMed]
- Tourasse C, Dénier JF, Awada A, Gratadour AC, Nessah-Bousquet K, Gay J. Elastography in the assessment of sentinel lymph nodes prior to dissection. Eur J Radiol 2012;81:3154-9. [Crossref] [PubMed]
- Youk JH, Son EJ, Kim JA, Gweon HM. Pre-Operative Evaluation of Axillary Lymph Node Status in Patients with Suspected Breast Cancer Using Shear Wave Elastography. Ultrasound Med Biol 2017;43:1581-6. [Crossref] [PubMed]
- Rautiainen S, Masarwah A, Sudah M, Sutela A, Pelkonen O, Joukainen S, Sironen R, Kärjä V, Vanninen R. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology 2013;269:54-60. [Crossref] [PubMed]
- Bhandari A, Xia E, Wang Y, Sindan N, Kc R, Guan Y, Lin YL, Wang X, Zhang X, Wang O. Impact of sentinel lymph node biopsy in newly diagnosed invasive breast cancer patients with suspicious node: a comparative accuracy survey of fine-needle aspiration biopsy versus core-needle biopsy. Am J Transl Res 2018;10:1860-73.
- Topps AR, Barr SP, Pikoulas P, Pritchard SA, Maxwell AJ. Pre-operative Axillary Ultrasound-Guided Needle Sampling in Breast Cancer: Comparing the Sensitivity of Fine Needle Aspiration Cytology and Core Needle Biopsy. Ann Surg Oncol 2018;25:148-53. [Crossref] [PubMed]
- Pyo JS, Jung J, Lee SG, Kim NY, Kang DW. Diagnostic Accuracy of Fine-Needle Aspiration Cytology and Core-Needle Biopsy in the Assessment of the Axillary Lymph Nodes in Breast Cancer-A Meta-Analysis. Diagnostics (Basel) 2020;10:717. [Crossref] [PubMed]


