
Treatment of intracranial aneurysms with pipeline embolization device: a single-center experience
Introduction
Intracranial aneurysm (IA) refers to an abnormal bulge in the blood vessels within the brain, with a prevalence of approximately 3.2% (1). Aneurysmal subarachnoid hemorrhage is associated with significant morbidity and mortality, with immediate fatality rates of 12% and more than 30% within a month if left untreated (2). Currently, endovascular treatments have become the primary method for managing IAs due to their advantages of minimal surgical invasion and improved neurological recovery (3,4).
In recent years, the introduction of flow diverter devices, particularly the pipeline embolization device (PED), has revolutionized endovascular treatments (5). The PED functions by modifying the hemodynamics of the IA and the artery carrying it, promoting the formation of endothelialization across the aneurysm neck, and ultimately leading to flow arrest, thrombosis, and occlusion within the aneurysm (6). The utilization of PEDs has expanded significantly, encompassing the treatment of distal circulation aneurysms, fusiform aneurysms, ruptured aneurysms, and aneurysms in the vertebrobasilar arteries (7-10).
While PEDs are primarily employed for the management of unruptured aneurysms (11,12), their use is not recommended for ruptured aneurysms due to the necessity of antiplatelet therapy prior to PED deployment, which may lead to secondary bleeding from the ruptured aneurysm (13,14). Additionally, the utilization of PEDs in the posterior circulation is relatively uncommon due to concerns regarding a higher incidence of procedure-related complications. This study aims to present our experience with PEDs in treating intracranial aneurysms (IA), including ruptured aneurysms and those in the posterior circulation, in order to contribute valuable insights into the use of PEDs for posterior circulation aneurysms and ruptured aneurysms. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1289/rc).
Methods
Study design and patient selection
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Independent Ethics Committee of the Fourth Affiliated Hospital of Xinjiang Medical University (No. 2022XE0149), and all patients provided written informed consent. The study collected demographic characteristics of the patients (age, gender, risk factors), morphological characteristics of the aneurysms (location and size), and comorbidities. Procedural details, immediate angiographic results, early and late complications, and follow-up outcomes were also recorded. Data collection was supervised by neurointervention experts. Aneurysms were classified according to maximum diameter, treatment method (isolated embolization or additive embolization), and location (anterior circulation or posterior circulation). Digital subtraction angiography (DSA) was used to evaluate aneurysm occlusion after PED placement and during follow-up, and O’Kelly-Marotta classification criteria were used for both angiographic images (15). If the PED was not successfully issued, the process was considered unsuccessful.
The number of PEDs inserted was determined by the operator, taking into consideration of the size and location of the aneurysm. The PEDs were delivered and deployed using either a Marksman microcatheter (Medtronic) or an XT27 microcatheter (Stryker). For patients with unruptured aneurysms, the antiplatelet regimen consisted of a combination of aspirin (100 mg daily) and clopidogrel (75 mg daily) for five consecutive days. The dosage of aspirin/clopidogrel was adjusted preoperatively based on thrombelastogram results. In the case of ruptured aneurysms, the antiplatelet regimen was modified for intraoperative use. Intravenous tirofiban was administered prior to PED release, followed by continuous tirofiban infusion for 24 hours postoperatively. After the patient regained consciousness, aspirin (100 mg daily) and clopidogrel (75 mg daily) were given orally, and tirofiban was discontinued after a 6-hour overlap with dual antiplatelet therapy (16).
Clinical and angiographic follow-up
The first angiographic follow-up was conducted at the 12-month postoperative mark (17). For patients whose aneurysms were not completely occluded during this initial follow-up, an additional follow-up visit was scheduled after six months.
Study outcomes
Complications involved the following (18): ischemic stroke [defined as a change in National Institutes of Health Stroke Scale (NIHSS) >4 lasting seven days or longer]; delayed aneurysmal rupture (DAR); distal parenchymal hemorrhage (DIPH); nerve compression symptoms (present or worsened after surgery); asymptomatic intracranial hemorrhage; aortic stenosis; standing exercise; death; (preoperative and immediate postoperative of less than 30 days).
The evaluations were carried out by two experienced senior neurointerventionalists. For this study, we engaged the expertise of two senior doctors, each with over 15 years of experience in diagnosing and treating relevant cases, to evaluate the imaging results of the intracranial aneurysm. In the event of any discrepancies in their evaluations, we sought the input of an arbitration expert, a doctor with over 20 years of relevant experience. The arbitration expert’s evaluation served as the final result.
Statistical analysis
Continuous variables were summarized as mean [standard deviation (SD)]. Categorical variables were expressed as frequencies (percentages). Data management and statistical analysis were performed in SPSS V.26.0.
Results
Baseline characteristics
From December 2018 to January 2022, a total of 60 consecutive patients with IAs were enrolled in this single-center study. The mean age of the patients was 61.8 years old, with 53.3% of them being female (Table 1). Among these patients, 12 (20%) had a history of subarachnoid hemorrhage prior to admission. Other comorbidities included hypertension in 21 patients (35%), diabetes mellitus in 17 patients (28.3%), and a current smoking history in 15 patients (25%). Regarding the size of the aneurysms, 34 cases (56.7%) had a diameter of less than 10 mm, 24 cases (40%) had a diameter between 10 and 25 mm, and 2 cases (3.3%) had a diameter greater than 25 mm. In terms of location, 54 cases (90%) were located in the anterior circulation, including four cases distal to the anterior circulation, while 6 cases (10%) were located in the posterior circulation, with four cases in the vertebral artery and two cases in the basilar artery.
Table 1
| Characteristics | Frequency |
|---|---|
| No. of patients | 60 |
| Age (years) | 61.8±10.4 |
| Women | 32 (53.3) |
| Current SAH | 12 (20.0) |
| Comorbidity | |
| Hypertension | 21 (35.0) |
| Hyperlipidemia | 14 (23.3) |
| Diabetes mellitus | 17 (28.3) |
| Cerebral atherosclerosis | 9 (15.0) |
| Smoking | |
| Never | 27 (45.0) |
| Previous | 18 (30.0) |
| Current | 15 (25.0) |
| Alcohol use | |
| Never | 36 (60.0) |
| Previous | 16 (26.7) |
| Current | 8 (13.3) |
| Presentation | |
| Headache | 21 (35.0) |
| Blepharoptosis or diplopia | 2 (3.3) |
| Vision impaired | 4 (6.6) |
| Asymptomatic | 33 (55.0) |
| Aneurysm size (max length, mm) | 11.2±6.2 |
| <10 | 34 (56.7) |
| 10–25 | 24 (40.0) |
| >25 | 2 (3.3) |
| Parent artery thrombosis | 2 (3.3) |
| Morphology | |
| Saccular | 49 (81.7) |
| Dissecting | 6 (10.0) |
| Fusiform | 4 (6.6) |
| Blister | 1 (1.7) |
| Width of neck (mm) | 4 (3.9±1.3) |
| Dome to neck ratio | 1.9 (2.0±0.7) |
| Aneurysm location | |
| Carotid artery | 50 (83.3) |
| Distal circle of Willis* | 4 (6.7) |
| Vertebral artery | 4 (6.7) |
| Basilar artery | 2 (3.3) |
| Intra-aneurysmal thrombus | 5 (8.3) |
Values are n (%) or mean ± SD. *, distal circle of Willis includes the middle cerebral artery, anterior cerebral artery, anterior communicating artery. Other posterior circulation includes posterior cerebral artery and posterior inferior cerebellar artery. SAH, subarachnoid hemorrhage; SD, standard deviation.
Procedural characteristics and angiographic outcomes
The treatment details and angiographic follow-up are presented in Table 2. Among the aneurysms, 53.3% were treated with PED alone, while 46.7% received adjunctive coiling. In the six patients with subarachnoid hemorrhage who received adjunctive coiling, the aneurysms were tightly filled. The average diameter of the PED device was 4.0±0.75 mm and the length was 22.7±5.3 mm. Technically, all patients had successful PED implantation at destination. Due to good compliance, all patients underwent DSA angiography at last follow-up. At last follow-up, 50 aneurysms (83.3%) achieved complete occlusion within a median of 13.0 months (range, 11–24 months). The overall complication rate was 3.3%, with no reported mortality.
Table 2
| Characteristics | Frequency (n=60) |
|---|---|
| Treatment method | |
| PED alone | 32 (53.3) |
| PED adjunctive coiling | 28 (46.7) |
| Loose packing | 22 (78.0) |
| Dense packing | 6 (22.0) |
| PED size | |
| Width (mm) | 4.0±0.75 |
| Length (mm) | 22.7±5.3 |
| Patients with multiple PED (n>1) | 1 (1.7) |
| Angiographic outcomes | 60 (100) |
| Device deployment to the target site | |
| Successful | 60 (100) |
| Placement failure or migration | 0 |
| Aneurysm satisfactory occlusion at last follow-up* | |
| Complete occlusion (D) | 50/60 (83.3) |
| Near-complete occlusion (C) | 6/60 (10.0) |
| Incomplete occlusion (A/B) | 4/60 (6.7) |
Values are n (%) or mean ± SD. *, 60 patients completed the last DSA follow-up. OKM grade: A complete occlusion (>95%); B partial occlusion (5–95%); C residual tumor neck (<5%); D not filled. PED, pipeline embolization device; SD, standard deviation; DSA, digital subtraction angiography; OKM, O’Kelly-Marotta.
Clinical outcomes
Table 3 presents the clinical outcomes. The patency rate of the parent artery was 98.3%. One case (1.7%) of in-stent stenosis occurred during follow-up, which resulted in an ischemic stroke in a patient who had been on dual antiplatelet therapy for only 2 months postoperatively. This was attributed to the closure of a branch vessel covered by the PED, as confirmed by angiographic follow-up. One patient with a ruptured aneurysm experienced a new postoperative neurological deficit. The overall complication rate was 3.3%, with no reported mortality.
Table 3
| Characteristics | Frequency (n=60) |
|---|---|
| Satisfactory status of parent arteries at follow-up | |
| Patency | 59 (98.3) |
| Stenosis | 1 (1.7) |
| Occlusion | 0 |
| Follow-up period | |
| Ischemic stroke | 1 (1.7) |
| DAR | 0 |
| DIPH | 0 |
| Recurrence rate | 0 |
| PED migration | 0 |
| mRS scores deteriorated | 1 (1.7) |
| Compressive symptoms at follow- up | 0 |
| Mortality | 0 |
| Total complication | 2 (3.3) |
| Total mortality | 0 |
Values are n (%). DAR, delayed aneurysmal rupture; DIPH, distal intraparenchymal hemorrhage; PED, pipeline embolization device; mRS, modified Rankin scale.
Subgroup analysis based on ruptured and unruptured aneurysms
Subgroup analysis based on ruptured and unruptured aneurysms is presented in Tables 4 and 5. The use of adjunctive coil embolization was significantly higher in ruptured aneurysms (100%) compared to unruptured aneurysms (33%). The total complication rate was significantly higher in the ruptured aneurysm group (16.6%) compared to the unruptured aneurysm group (0%), and the rate of complete occlusion at follow-up was higher in ruptured aneurysms (91.7%) compared to unruptured aneurysms (81.3%).
Table 4
| Characteristics | Ruptured (n=12) | Unruptured (n=48) |
|---|---|---|
| Age (years) | 63.1±9.5 | 61.5±10.4 |
| Woman | 7 (58.3) | 25 (52.0) |
| Aneurysm size (max length, mm) | 8.2±1.0 | 11.9±6.7 |
| <10 | 8 (66.7) | 26 (54.2) |
| 10–25 | 4 (33.3) | 20 (41.7) |
| >25 | 0 | 2 (4.1) |
| Treatment | ||
| PED alone | 0 | 32 (66.7) |
| PED adjunctive coiling | 12 (100) | 16 (33.3) |
| PED size, mm | ||
| Width | 4.2±0.47 | 4.0±0.8 |
| Length | 19.8±2.8 | 23.4±5.6 |
| Patients with multiple PED (n>1) | 1 (8.3) | 0 |
| Stenosis | 1 (8.3) | 0 |
| Ischemic stroke | 1 (8.3) | 0 |
| mRS scores deteriorated | 1 (8.3) | – |
| Total complication | 2 (16.6) | 0 |
| Total mortality | 0 | 0 |
| Complete occlusion (D) | 11 (91.7) | 39 (81.3) |
| Near-complete occlusion (C) | 1 (8.3) | 5 (10.4) |
| Incomplete occlusion (A/B) | 0 | 4 (8.3) |
Values are n (%) or mean ± SD. OKM grade: A, complete occlusion (>95%); B, partial occlusion (5–95%); C, residual tumor neck (<5%); D, not filled. SD, standard deviation; PED, pipeline embolization device; mRS, modified Rankin scale; OKM, O’Kelly-Marotta.
Table 5
| Characteristics | Posterior circulation (n=6) | Anterior circulation (n=54) |
|---|---|---|
| Age (years) | 61.3±10.2 | 66.3±9.6 |
| Woman | 2 (33.3) | 30 (55.6) |
| Aneurysm size (max length, mm) | 8.0±3.0 | 11.5±6.4 |
| <10 | 4 (66.7) | 30 (55.6) |
| 10–25 | 1 (16.7) | 23 (42.6) |
| >25 | 1 (16.7) | 1 (1.8) |
| Treatment | ||
| PED alone | 4 (66.7) | 28 (51.9) |
| PED adjunctive coiling | 2 (33.3) | 26 (48.1) |
| PED size, mm | ||
| Width | 4.0±0.75 | 4.0±0.79 |
| Length | 22.5±5.19 | 24.3±7.00 |
| Patients with multiple PED (n>1) | ||
| Ruptured | 0 | 12 (22) |
| Stenosis | 0 | 1 (1.9) |
| Ischemic stroke | 0 | 1 (1.9) |
| mRS scores deteriorated | 0 | 1 (1.9) |
| Total complication | – | 2 (3.7) |
| Total mortality | ||
| Complete occlusion (D) | 5 (83.3) | 45 (83.3) |
| Near-complete occlusion (C) | 1 (16.7) | 5 (9.3) |
| Incomplete occlusion (A/B) | 0 | 4 (7.4) |
Values are n (%) or mean ± SD. OKM grade: A, complete occlusion (>95%); B, partial occlusion (5–95%); C, residual tumor neck (<5%); D, not filled. SD, standard deviation; PED, pipeline embolization device; mRS, modified Rankin scale; OKM, O’Kelly-Marotta.
Illustrative case
Case 1
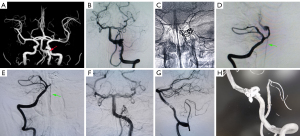
The patient’s cranial magnetic resonance angiography (MRA) suggested an aneurysm, involving the union of the vertebrobasilar artery (Figure 1). Orthotopic cerebral angiogram of the aneurysm was as follows: a spring coil was used to loosely occlude the aneurysm, and a PED was placed. The aneurysm accumulated the basilar artery and the right vertebral artery, and the right vertebral artery was occluded using the coil. The imaging suggested occlusion of the right vertebral artery with blurred visualization of the basilar artery, green arrow suggests anterior spinal artery. DSA follow-up at the 13th month postoperatively suggested that the right vertebral artery was completely occluded, and the anterior spinal artery was well visualized (green arrow); DSA follow-up at the 13th month postoperatively suggested that the aneurysm was completely occluded, and the aneurysm-carrying artery was patent and the branches were patent: 13-month postoperative three-dimensional (3D) imaging showed complete occlusion of the aneurysm and patency of the aneurysm-carrying artery.
Case 2
Figure 2 demonstrates a ruptured blood blister aneurysm of the right cavernous segment. Preoperative 3D reconstruction showed ruptured blood blister aneurysms. During the release of the PED, the embolization microcatheter was displaced and pointed into the conus arteriosus below. A 2–4 coil was filled into the conus arteriosus. Intraoperative angiography showed that the position and adherence of the stent were good. Angiography was performed 12 months postoperation, the 3D reconstruction showed that the blood blister aneurysm healed perfectly.

Discussion
This single-center observational study provides evidence supporting the safe and effective use of PEDs for the treatment of IAs, irrespective of their morphology and location. Our study included 60 consecutive patients with 60 IA who underwent PED treatment. The median duration of follow-up was 13.0 months (range, 11–24 months). All patients underwent final DSA angiographic follow-up, and a high rate of complete occlusion (83.3%) was achieved. The overall rates of complications and mortality were 3.3% and 0%, respectively. Specifically, in the subgroup of ruptured aneurysms treated with PEDs, we observed a satisfactory rate of complete occlusion (91.7%). However, it is important to note that the complication rate (16.6%) associated with this approach in ruptured aneurysms remains significant and should not be overlooked.
Safety and efficiency of PED treatment for ruptured IA
We present the treatment process and outcomes of a series of patients with ruptured aneurysms who were treated off-label with PEDs. A meta-analysis conducted by Cagnazzo et al. (19) comparing findings from other studies reported a 7.1% incidence of in-stent thrombosis and permanent complications when PEDs were used for treating ruptured aneurysms. Additionally, the analysis showed an 85.7% likelihood of adjunctive coil usage, which aligns with our own findings. Despite the limited data available, we achieved favorable treatment outcomes. However, further investigation is warranted to better understand the efficacy and safety of this technique. Zammar et al. (20) conducted a study on ruptured aneurysms treated with PEDs and reported a complete occlusion rate of 82.5%. The study also identified the use of PEDs for ruptured aneurysms as an independent predictor of postoperative decline in modified Rankin scale (mRS) scores, and demonstrated that aneurysm size independently predicted complete occlusion when PEDs were used off-label.
In our study, we treated ruptured aneurysms with PEDs, and 12 patients underwent adjunctive coiling, resulting in a 91.7% complete occlusion rate (according to the O’Kelly-Marotta grading system). The use of PED-assisted coils in the treatment of ruptured aneurysms showed higher occlusion rates compared to conventional stent-assisted coil therapy (21). We attribute this difference to the greater coverage provided by PEDs and our careful patient selection process. In a study by Foreman et al. (22) involving the use of PEDs for ruptured aneurysms, we achieved better effectiveness and lower complication rates compared to the Foreman et al.’s findings. Bodily et al. (23), in a study on stent-assisted embolization for IA, reported higher rates of hemorrhagic and thromboembolic complications in treated ruptured aneurysms compared to unruptured aneurysms. Our findings align with this observation, and considering the higher complication rates associated with ruptured aneurysms, we believe that making informed treatment choices is crucial in effectively reducing the complication rate. Factors such as the decision to use adjunctive coil embolization and the choice between PEDs and regular stents should be carefully considered (24,25).
Safety and efficiency of PED treatment for posterior circulation aneurysms
The prognosis of posterior circulation aneurysms after PED treatment is primarily influenced by factors such as the morphology, location, size, and history of rupture of the aneurysm. In a multicenter study conducted in North America and Europe by Griessenauer et al. (26), the highest rate of complete occlusion was observed in dissecting aneurysms, followed by saccular and fusiform aneurysms. The study suggested that the neurovascular structure of coarctation aneurysms may be more amenable to remodeling blood flow (27,28). Smaller size, dissecting or saccular morphology posterior circulation aneurysms have good rates of complete or near-complete occlusion, while saccular aneurysms of the basilar artery have the lowest occlusion rates compared to saccular aneurysms in other locations (29).
Compared to anterior circulation aneurysms, the use of PEDs for posterior circulation aneurysms is currently considered off-label. As a result, we exercised greater caution in evaluating and selecting patients for this treatment approach. For example, the two basilar artery aneurysms included in our study were located in the inferior segment of the basilar artery, which is considered a relatively safer position for off-label use of PEDs in the vertebral basilar artery (30,31). The most recent study (26) showed no significant effect of intra-aneurysmal thrombus, the number of PEDs placed, or the use of adjunctive coils on aneurysm occlusion, which differs from previous findings. The six unruptured vertebrobasilar artery aneurysms in our study, two of which were fusiform aneurysms and four were saccular aneurysms, all demonstrated a good prognosis after treatment. Therefore, we believe that the use of PEDs for unruptured aneurysms is safer and more effective, but careful preoperative evaluation is required to ensure optimal patient selection.
Limitations
There are several limitations that should be acknowledged and taken into consideration when interpreting the results. Firstly, the study design is retrospective and conducted at a single center, which introduces the possibility of bias and limits the generalizability of the findings. To address this, the researchers performed additional subgroup analyses to mitigate potential biases.
Another limitation is the heterogeneity of the patient sample, which includes a small proportion of patients with vertebrobasilar aneurysms (6 out of 60). This limited representation of vertebrobasilar aneurysms may affect the overall conclusions and generalizability of the findings to this specific subgroup.
Finally, in our study, the percentage of patients with complete occlusion at baseline and follow-up was relatively low, limiting the ability to assess the duration of intervention and long-term outcomes.
Conclusions
In conclusion, this study demonstrates that PED may be an effective treatment option for IA with good occlusion rates and low complication rates. However, caution is still necessary when applying PEDs for the treatment of ruptured aneurysms due to the higher complication rate associated with this condition. Regarding the treatment of vertebrobasilar artery aneurysms with PEDs, the study showed promising efficacy without complications nor patient deaths, but further research is needed to validate these findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1289/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1289/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Independent Ethics Committee of the Fourth Affiliated Hospital of Xinjiang Medical University (No. 2022XE0149), and all patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pontes FGB, da Silva EM, Baptista-Silva JC, Vasconcelos V. Treatments for unruptured intracranial aneurysms. Cochrane Database Syst Rev 2021;5:CD013312. [Crossref] [PubMed]
- Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet 2022;400:846-62. [Crossref] [PubMed]
- Gao P, Jin Z, Wang P, Zhang X. Effects of Intracranial Interventional Embolization and Intracranial Clipping on the Cognitive and Neurologic Function of Patients with Intracranial Aneurysms. Arch Clin Neuropsychol 2022;37:1688-98. [Crossref] [PubMed]
- Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP, Cruz-Flores S. Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke 2010;41:1471-6. [Crossref] [PubMed]
- Hanel RA, Cortez GM, Lopes DK, Nelson PK, Siddiqui AH, Jabbour P, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device (PREMIER study): 3-year results with the application of a flow diverter specific occlusion classification. J Neurointerv Surg 2023;15:248-54. [Crossref] [PubMed]
- Fiorella D, Lylyk P, Szikora I, Kelly ME, Albuquerque FC, McDougall CG, Nelson PK. Curative cerebrovascular reconstruction with the Pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg 2018;10:i9-i18. [Crossref] [PubMed]
- Ravindran K, Enriquez-Marulanda A, Kan PTM, Renieri L, Limbucci N, Mangiafico S, Salem MM, Alturki AY, Moore JM, Ogilvy CS, Thomas AJ. Use of Flow Diversion for the Treatment of Distal Circulation Aneurysms: A Multicohort Study. World Neurosurg 2018;118:e825-33. [Crossref] [PubMed]
- Griessenauer CJ, Enriquez-Marulanda A, Xiang S, Hong T, Zhang H, Taussky P, et al. Comparison of PED and FRED flow diverters for posterior circulation aneurysms: a propensity score matched cohort study. J Neurointerv Surg 2021;13:153-8. [Crossref] [PubMed]
- Griessenauer CJ, Enriquez-Marulanda A, Taussky P, Biswas A, Grandhi R, Xiang S, et al. Experience With the Pipeline Embolization Device for Posterior Circulations Aneurysms: A Multicenter Cohort Study. Neurosurgery 2020;87:1252-61. [Crossref] [PubMed]
- Turhon M, Kang H, Li M, Liu J, Zhang Y, Zhang Y, et al. Treatment of fusiform aneurysms with a pipeline embolization device: a multicenter cohort study. J Neurointerv Surg 2023;15:315-20. [Crossref] [PubMed]
- Enriquez-Marulanda A, Penumaka A, Ogilvy CS, Thomas AJ, Moore JM. Safety and Efficacy of the Off-Label Use of Pipeline Embolization Device Based on the 2018 Food and Drug Administration-Approved Indications for Intracranial Aneurysms: A Single-Center Retrospective Cohort Study. Neurosurgery 2022;90:700-7. [Crossref] [PubMed]
- Hanel RA, Kallmes DF, Lopes DK, Nelson PK, Siddiqui A, Jabbour P, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg 2020;12:62-6. [Crossref] [PubMed]
- Tawk RG, Hasan TF, D'Souza CE, Peel JB, Freeman WD. Diagnosis and Treatment of Unruptured Intracranial Aneurysms and Aneurysmal Subarachnoid Hemorrhage. Mayo Clin Proc 2021;96:1970-2000. [Crossref] [PubMed]
- Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, Maiuri F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol J 2015;28:365-75. [Crossref] [PubMed]
- O'kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010;16:133-7. [Crossref] [PubMed]
- Samaniego EA, Gibson E, Nakagawa D, Ortega-Gutierrez S, Zanaty M, Roa JA, Jabbour P, Hasan DM. Safety of tirofiban and dual antiplatelet therapy in treating intracranial aneurysms. Stroke Vasc Neurol 2019;4:36-42. [Crossref] [PubMed]
- Turhon M, Kang H, Liu J, Zhang Y, Zhang Y, Huang J, et al. In-Stent Stenosis After Pipeline Embolization Device in Intracranial Aneurysms: Incidence, Predictors, and Clinical Outcomes. Neurosurgery 2022;91:943-51. [Crossref] [PubMed]
- Kang H, Zhou Y, Luo B, Lv N, Zhang H, Li T, Song D, Zhao Y, Guan S, Maimaitili A, Wang Y, Feng W, Wang Y, Wan J, Mao G, Shi H, Yang X, Liu J. Pipeline Embolization Device for Intracranial Aneurysms in a Large Chinese Cohort: Complication Risk Factor Analysis. Neurotherapeutics 2021;18:1198-206. [Crossref] [PubMed]
- Cagnazzo F, Di Carlo DT, Petrella G, Perrini P. Ventriculostomy-related hemorrhage in patients on antiplatelet therapy for endovascular treatment of acutely ruptured intracranial aneurysms. A meta-analysis. Neurosurg Rev 2020;43:397-406. [Crossref] [PubMed]
- Zammar SG, Buell TJ, Chen CJ, Crowley RW, Ding D, Griessenauer CJ, Hoh BL, Liu KC, Ogilvy CS, Raper DM, Singla A, Thomas AJ, Cockroft KM, Simon SD. Outcomes After Off-Label Use of the Pipeline Embolization Device for Intracranial Aneurysms: A Multicenter Cohort Study. World Neurosurg 2018;115:e200-5. [Crossref] [PubMed]
- Gory B, Blanc R, Turjman F, Berge J, Piotin M. The Barrel vascular reconstruction device for endovascular coiling of wide-necked intracranial aneurysms: a multicenter, prospective, post-marketing study. J Neurointerv Surg 2018;10:969-74. [Crossref] [PubMed]
- Foreman PM, Ilyas A, Cress MC, Vachhani JA, Hirschl RA, Agee B, Griessenauer CJ. Ruptured Intracranial Aneurysms Treated with the Pipeline Embolization Device: A Systematic Review and Pooled Analysis of Individual Patient Data. AJNR Am J Neuroradiol 2021;42:720-5. [Crossref] [PubMed]
- Bodily KD, Cloft HJ, Lanzino G, Fiorella DJ, White PM, Kallmes DF. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol 2011;32:1232-6. [Crossref] [PubMed]
- Zheng Y, Liu Y, Leng B, Xu F, Tian Y. Periprocedural complications associated with endovascular treatment of intracranial aneurysms in 1764 cases. J Neurointerv Surg 2016;8:152-7. [Crossref] [PubMed]
- Kang H, Luo B, Liu J, Zhang H, Li T, Song D, Zhao Y, Guan S, Maimaitili A, Wang Y, Feng W, Wang Y, Wan J, Mao G, Shi H, Wang K, Yang X. Mortality after treatment of intracranial aneurysms with the Pipeline Embolization Device. J Neurointerv Surg 2022;14:neurintsurg-2020-017002.
- Griessenauer CJ, Ogilvy CS, Adeeb N, Dmytriw AA, Foreman PM, Shallwani H, et al. Pipeline embolization of posterior circulation aneurysms: a multicenter study of 131 aneurysms. J Neurosurg 2018;130:923-35. [Crossref] [PubMed]
- Albuquerque FC, Park MS, Abla AA, Crowley RW, Ducruet AF, McDougall CG. A reappraisal of the Pipeline embolization device for the treatment of posterior circulation aneurysms. J Neurointerv Surg 2015;7:641-5. [Crossref] [PubMed]
- Bhogal P, Pérez MA, Ganslandt O, Bäzner H, Henkes H, Fischer S. Treatment of posterior circulation non-saccular aneurysms with flow diverters: a single-center experience and review of 56 patients. J Neurointerv Surg 2017;9:471-81. [Crossref] [PubMed]
- de Barros Faria M, Castro RN, Lundquist J, Scrivano E, Ceratto R, Ferrario A, Lylyk P. The role of the pipeline embolization device for the treatment of dissecting intracranial aneurysms. AJNR Am J Neuroradiol 2011;32:2192-5. [Crossref] [PubMed]
- Wang J, Jia L, Duan Z, Wang Z, Yang X, Zhang Y, Lv M. Endovascular Treatment of Large or Giant Non-saccular Vertebrobasilar Aneurysms: Pipeline Embolization Devices Versus Conventional Stents. Front Neurosci 2019;13:1253. [Crossref] [PubMed]
- Zhou Y, Wu X, Tian Z, Yang X, Mu S. Pipeline Embolization Device With Adjunctive Coils for the Treatment of Unruptured Large or Giant Vertebrobasilar Aneurysms: A Single-Center Experience. Front Neurol 2020;11:522583. [Crossref] [PubMed]


