
Traumatic neuromas with hemangiomas of diverse morphology in the left upper arm: a case description
Introduction
Traumatic neuroma is a benign tumor of unknown etiology suspected to be caused by trauma, injury, or surgery (1). Intraneural hemangiomas are rare benign mesodermal tumors (2). However, traumatic neuromas with hemangiomas have not been reported in the relevant literature. Herein, we reported the unique occurrence of the bulbous and spindle forms of a traumatic neuroma with hemangioma in a patient who had a previous infection and underwent amputation.
Case presentation
A 78-year-old woman, who had undergone amputation of the left forearm due to soft tissue infection, noticed an incidental mass of approximately 10×3 cm in size on the stump of the left upper limb three months earlier, which was accompanied by occasional pain from traction without fever, redness, swelling, nor pus.
She visited our department because of the increased frequency of pain episodes; her family history was unremarkable.
Clinical examination revealed no acute distress. Her left upper arm exhibited a healing stump with a subcutaneous mass of approximately 10×3 cm in length on the medial and lateral sides of the upper arm, which was soft, slightly tender on palpation, with clear boundaries, no obvious adhesion to surrounding tissues, and a good range of motion; no sign of ulceration or inflammation was present.
Laboratory examination: glucose: 6.34 mmol/L (normal: 3.90–5.90 mmol/L), alkaline phosphatase: 138 U/L (normal: 50–135 U/L), cytokeratin-19-fragment: 7.13 ng/mL (normal: 0–5 ng/mL).
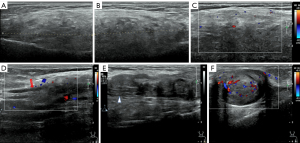
B-mode ultrasound (US) revealed an ill-defined homogeneously isoechoic fusiform mass on the left upper lateral arm (Figure 1). On color-Doppler (CD), few vascular signals were detected inside and around the mass (Figure 1C). Additionally, a heterogeneously hypoechoic marginated bar mass with echogenic patches and ‘bulbous distal end’ morphology on the medial left upper arm with its proximal end appearing to be in continuity with a normal nerve proximally was revealed (Figure 1E). On CD, vascular signals were detected inside and around the mass (Figure 1F). A longitudinal B-mode scan confirmed that the oval mass did not infiltrate the muscular fascia. These findings suggested a schwannoma.
Magnetic resonance imaging (MRI) (3.0 tesla resonance magnetic imager) revealed a well-defined strip-like subcutaneous lesion with a hypointensity and heterogeneous intermediate-hyperintensity with a typical floccular pattern on T1- and T2-weighted images (WIs), respectively (Figure 2). The upper end of the mass extended to the left ulnar nerve (Figure 2C,2D). In the intermuscular space in front of the humerus, we observed an ill-defined ovoid lesion with a heterogeneously hypointensity and heterogeneous intermediate-hyperintensity with a typical floccular pattern on T1- and T2-WIs, respectively. The lesion followed the surface (Figure 2H,2I) and partially surrounded the lower segment of the humerus with a coarse margin with no clear abnormal signal in the bone marrow. Additionally, the spectral attenuated inversion-recovery (SPAIR) T2-WIs did not reveal significant signal reduction for both the lesions, and a vivid heterogeneous enhancement was observed in the contrast-enhanced fat-saturated T1-WIs. These MRI findings suggested malignancies of mesenchymal origin, specifically undifferentiated pleomorphic sarcomas (UPSs), which must be differentiated from malignant schwannomas.

The patient underwent complete tumor resection with additional margin clearance under general anesthesia, because the surgeons believed the degree of damage to the humerus was mild. In the lateral upper arm, a heterogeneous spindle lump was identified, measuring approximately 10 cm × 5 cm, lacking envelope, with unclear boundary, from the median nerve and the radial nerve of the upper arm. During sharp blunt dissection, the lump was observed to be close to the eroded humeral surface, and some bone fragments were detached. Thus, the median and radial nerves were severed at their point of origin. Subsequently, a longitudinal uneven lump of approximately 13 cm was observed on the medial side of the upper arm, which was well encapsulated with clear boundaries surrounding the brachial artery and ulnar nerve in the upper arm. The ulnar nerve was severed and the lump was separated at its origin. Simultaneously, the brachial artery was ligated. The resected specimens were sent for histopathological examination. Pathological results (Figure 3) confirmed that both masses were amputation neuromas with hemangiomas and exhibited signs of intratumoral hemorrhages.

The patient’s postoperative recovery was uneventful. No symptom recurred during the 1.5-year follow-up period.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
A traumatic neuroma is a chaotic accumulation of fibroneural tissue that develops due to an unsuccessful attempt at nerve regeneration after injury (3) Histologically, a traumatic neuroma is a non-encapsulated and non-neoplastic amalgamation of cells and axons encased within a dense fibrotic matrix (4). The most common presenting symptoms are pain, numbness, discomfort, and symptoms resembling electrical shocks, but approximately 20–30% of all neuromas are painful (5,6). When a somatic cause of pain is detected, its corresponding treatment can alleviate the pain (7). However, a definitive treatment modality for the management of painful neuromas is yet to be established (8). Previous studies have indicated that nerve capping technique, which allows for epineural healing over the severed fascicles within the chamber (9), is now a useful option for the treatment or prevention of neuromas (10). Traumatic neuromas are severe nerve injuries that impair conduction. They are classified as neuroma-in-continuity (NIC) after partial nerve transection or end-bulb neuromas (EBN) after complete disruption (11). Traumatic neuromas exhibit proximal continuity with the parent nerve, analogous to a ‘tail sign’ associated with peripheral nerve sheath tumors (PNSTs), indicating their neurogenic origin. However, EBNs lack distal continuity with the parent nerve, while NICs are contiguous both proximally and distally. A neuroma is typically an oval hypoechoic mass with a diameter larger than a normal nerve on the US (12). The relationship between tumors and nerve bundles can be distinguished by ultra-high-frequency ultrasound (UHFUS) (13), a novel technique capable of accessing structures up to 30 µm. Amputation neuromas are generally T2 heterogeneous lesions with high signal intensity and are observed as end-bulb or spindle-type lesions. MRI can reveal enlarged fascicles, dark outer rims, and varying levels of enhancement. The heterogeneity of T2-weighted imaging is an independent predictor of symptomatic neuromas (14).
Intraneural hemangioma is an extremely rare neoplasm that develops within the endothelium of the nerve. New blood vessels originate from the endoneurium or wrap around the nerve and its surrounding tissues. Histologically, intraneural hemangiomas are usually intertwined with nerve bundles and are rich in nerve fiber bundles. The components of the tumor are closely related to the nerves and usually present with pain, nerve compression, and nerve entrapment symptoms (15,16). On US, intraneural hemangiomas manifest as well-circumscribed hypoechoic forms with posterior acoustic enhancement. On MRI, these tumors exhibit areas of hyperintensity on T1- and T2-weighted fat-suppressed sequences, although the T1 appearance may vary due to the presence of flow voids, feeding veins, and draining vessels. Classically, there is enhancement observed in post-contrast gadolinium images (17-19).
Traumatic neuromas combined with hemangiomas have rarely been reported and their imaging characteristics remain unclear. However, according to the pathogenesis of neuroma and intraneural hemangioma, we can speculate that the occurrence of traumatic neuroma with hemangioma is due to disrupted injured nerve repair and abnormal growth of neovascularization in the nerve endothelium. Therefore, it may have the characteristics of both the tumors simultaneously.
We reported a case of an aged female patient who discovered a mass on the left upper limb stump after amputation, occasionally accompanied by traction pain. US revealed two hypoechoic masses in the stump of the left upper arm, with one appearing connected to the normal nerve. MRI revealed heterogeneous high-signal tumors on T2-WIs, with clear or unclear boundaries and marked enhancement on contrast-enhanced scans. The mass in the left lateral upper arm eroded the adjacent bone cortex, which is consistent with the imaging findings of malignant tumors. UPS is the most common soft tissue malignancy in middle-aged and elderly people. Radiographically, UPS appears as large soft-tissue masses, typically adjacent to long bone diaphyses in the extremities. Computed tomography (CT) revealed that the mass contained areas of decreased attenuation, reflecting myxomatous tissue, hemorrhage, and/or necrosis. CT can be used to detect internal calcifications and adjacent osseous cortical erosion or invasion. The MR characteristics are nonspecific, usually demonstrating an isointensity on T1-WIs and a heterogeneous hyperintensity on T2-WIs. Myxoid components can demonstrate areas of low T1- and high T2-weighted signal intensity. An increase in the solid components was observed. UPS may spread from the dominant mass in a ‘tail like’ fashion, which may indicate aggressive regional infiltration at presentation (20). The misdiagnosis of these tumors as malignant is understandable because they are different from typical neuromas, in addition to being continuous with the proximal normal nerve and presence of ‘enlarged fascicles’. In this case, large and soft masses, cortical erosion around the tumor, heterogeneous signals, and marked enhancement after enhancement on MRI may suggest these are not simple neuromas. The presence of multiple small blood vessels around the tumor extending into it may indicate the presence of a hemangioma, although hemangiomas typically do not have a capsule. The distinct biological behaviors of both the tumors in the left upper arm were attributed to the vascular components in the tumor and the timing of fibrous envelope formation. In this case, the intermediate to bright signals in T2-WIs with fat-suppression indicated cellular nerve fascicles, peripheral dark signals representing a collagenous matrix (20-22), and the dark outer rim indicated the fibrous envelope. In addition, mixed flocculent signals may reflect blood sinuses. A clinical history of trauma and the lack of target signs on imaging may be the most useful clues in the diagnosis.
Radiological imaging is necessary to identify the origin of soft tissue lesions and determine its malignancy (23). Imaging techniques, including US and MRI, are the best modalities for characterizing these lesions and have a direct influence on the appropriate management and treatment (23).
This case should be differentiated from the following diseases. UPS is the most common soft tissue sarcoma without enhanced divider sign and extensive soft tissue edema around the tumor body. Schwannomas are the most common benign neurogenic tumors with a small size, well-defined boundaries, and the presence of signs of tail, target, and split fat (24). Malignant peripheral nerve sheath tumors (MPNSTs) are rare soft tissue sarcomas mostly related to neurofibromatosis type-1 (NF1) and benign peripheral nerve sheath tumors (BPNSTs). MPNSTs are large, with ill-defined margins, possibility of invading adjacent structures, and a lack of specific contact with adjacent nerves (24).
Conclusions
In the present case, the initial MRI impression was suggestive of UPS. However, the biopsy revealed a traumatic neuroma with hemangioma, which is a rare occurrence. This case highlights the importance of considering a traumatic neuroma with hemangioma as a differential diagnosis in patients who exhibit continuous, painful, and aggressive lesions with the normal proximal nerve on MRI following amputation.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1590/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Foltán R, Klíma K, Spacková J, Sedý J. Mechanism of traumatic neuroma development. Med Hypotheses 2008;71:572-6. [Crossref] [PubMed]
- Zargarbashi R, Vosoughi F, Milan N. Wide resection as a solution to excruciating pain in intraneural hemangioma: Follow-up of a previously published case report. Int J Surg Case Rep 2022;98:107562. [Crossref] [PubMed]
- SWANSON HH. Traumatic neuromas. A review of the literature. Oral Surg Oral Med Oral Pathol 1961;14:317-26. [Crossref] [PubMed]
- Mathews GJ, Osterholm JL. Painful traumatic neuromas. Surg Clin North Am 1972;52:1313-24. [Crossref] [PubMed]
- Kang J, Yang P, Zang Q, He X. Traumatic neuroma of the superficial peroneal nerve in a patient: a case report and review of the literature. World J Surg Oncol 2016;14:242. [Crossref] [PubMed]
- Krishnan KG, Pinzer T, Schackert G. Coverage of painful peripheral nerve neuromas with vascularized soft tissue: method and results. Neurosurgery 2005;56:369-78; discussion 369-78. [Crossref] [PubMed]
- Lu C, Sun X, Wang C, Wang Y, Peng J. Mechanisms and treatment of painful neuromas. Rev Neurosci 2018;29:557-66. [Crossref] [PubMed]
- Chou J, Liston JM, DeGeorge BR. Traditional Neuroma Management Strategies: A Systematic Review. Ann Plast Surg 2023;90:S350-5. [Crossref] [PubMed]
- Yan H, Zhang F, Kolkin J, Wang C, Xia Z, Fan C. Mechanisms of nerve capping technique in prevention of painful neuroma formation. PLoS One 2014;9:e93973. [Crossref] [PubMed]
- Sisti A, Uygur S, Lopez-Schultz SD, Konofaos P. Nerve Capping Techniques for Neuroma Management: A Comprehensive Literature Review. Ann Plast Surg 2024;92:106-19. [Crossref] [PubMed]
- Chhabra A, Williams EH, Wang KC, Dellon AL, Carrino JA. MR neurography of neuromas related to nerve injury and entrapment with surgical correlation. AJNR Am J Neuroradiol 2010;31:1363-8. [Crossref] [PubMed]
- Rajput K, Reddy S, Shankar H. Painful neuromas. Clin J Pain 2012;28:639-45. [Crossref] [PubMed]
- Forte AJ, Boczar D, Oliver JD, Sisti A, Clendenen SR. Ultra-high-frequency Ultrasound to Assess Nerve Fascicles in Median Nerve Traumatic Neuroma. Cureus 2019;11:e4871. [Crossref] [PubMed]
- Chung BM, Lee GY, Kim WT, Kim I, Lee Y, Park SB. MRI features of symptomatic amputation neuromas. Eur Radiol 2021;31:7684-95. [Crossref] [PubMed]
- Pulidori M, Capuano C, Mouchaty H, Cioffi F, Di Lorenzo N. Intramuscular thrombosed arteriovenous hemangioma of the upper right arm mimicking a neuroma of the ulnar nerve: case report. Neurosurgery 2004;54:770-1; discusion 771-2.
- Vekris MD, Stafilas KS, Zacharis KX, Xenakis TA, Soucacos PN, Beris AE. Intrinsic haemangioma of the median nerve: report of a case and review of the literature. Microsurgery 2008;28:89-90. [Crossref] [PubMed]
- Al-Garnawee M, Najjar M. Median Nerve Cavernous Hemangioma. Basic Clin Neurosci 2017;8:255-9. [Crossref] [PubMed]
- Bacigaluppi S, Fiaschi P, Prior A, Bragazzi NL, Merciadri P, Gennaro S. Intraneural haemangioma of peripheral nerves. Br J Neurosurg 2020;34:480-6. [Crossref] [PubMed]
- Doğramaci Y, Kalaci A, Sevinç TT, Yanat AN. Intraneural hemangioma of the median nerve: A case report. J Brachial Plex Peripher Nerve Inj 2008;3:5. [Crossref] [PubMed]
- Alpert JS, Boland P, Hameed M, Panicek DM. Undifferentiated pleomorphic sarcoma: indolent, tail-like recurrence of a high-grade tumor. Skeletal Radiol 2018;47:141-4. [Crossref] [PubMed]
- Oliveira KMC, Pindur L, Han Z, Bhavsar MB, Barker JH, Leppik L. Time course of traumatic neuroma development. PLoS One 2018;13:e0200548. [Crossref] [PubMed]
- Singson RD, Feldman F, Staron R, Fechtner D, Gonzalez E, Stein J. MRI of postamputation neuromas. Skeletal Radiol 1990;19:259-62. [Crossref] [PubMed]
- Tagliafico AS, Isaac A, Bignotti B, Rossi F, Zaottini F, Martinoli C. Nerve Tumors: What the MSK Radiologist Should Know. Semin Musculoskelet Radiol 2019;23:76-84. [Crossref] [PubMed]
- Chhabra A, Soldatos T, Durand DJ, Carrino JA, McCarthy EF, Belzberg AJ. The role of magnetic resonance imaging in the diagnostic evaluation of malignant peripheral nerve sheath tumors. Indian J Cancer 2011;48:328-34. [Crossref] [PubMed]


