
Diagnosis of lupus mastitis via multimodal ultrasound: case description
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that primarily afflicts women of childbearing age and can affect any organ or system in the body. The main clinical features include the presence of multiple autoantibodies, such as antinuclear antibodies, and the involvement of various organs and tissues. If a patient with SLE presents with a fever, this can indicate disease activity. Lupus panniculitis (LP) is a rare type of SLE, occurring in only 2% of those with SLE. It typically affects the upper arm, shoulder, face, and buttocks. Lupus mastitis (LM) is a rare manifestation of LP, characterized by inflammation of the deep subcutaneous fatty tissue of the breast, dense lymphatic plasma cell infiltration, and transparent fat necrosis in the lobules. It is most common in middle-aged women, often affecting both breasts, and its cause is not yet clear (1). We report a case in which ultrasonography of a mass was characterized by a low echo, unclear boundary, and irregular shape, with a coarse and robust solid echo inside the mass and color Doppler ultrasound showing a short strip of blood flow signal around the mass; cumulatively, these findings led to a misdiagnose of malignant tumor. The purpose of reporting this case is to prevent future misdiagnosis and missed diagnosis by explaining how combining the history of the lesions with the characteristics of this case is more informative than solely relying on ultrasound characteristics.
Case presentation
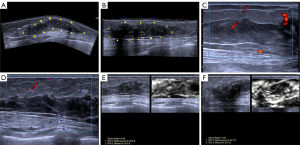
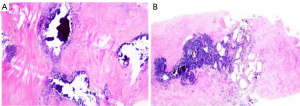
A 26-year-old female patient inadvertently found a mass in the bilateral breast a week before visiting our center but had no fever, pain, local redness, ulceration, exudation, or nipple discharge. She had SLE for 9 years and had been undergoing long-term hormone therapy, with her condition being stable over the previous 2 years. She denied any contact with toxic substances or a history of radiation exposure. The patient was treated in other hospitals and did not experience improvement after treatment, so she came to Gansu Provincial Maternity and Child-care Hospital for further treatment. Two-dimensional ultrasound showed irregular hypoechoic areas around both areolas, measuring 85×13 mm in size in the right breast (Figure 1A) and 52×13 mm in the left breast, respectively (Figure 1B), along with poorly defined borders and coarse, strong echoes. The ultrasound findings of irregular bilateral breast hypoechoic areas was consistent with LM and Breast Imaging-Reporting and Data System (BI-RADS) 4. Color Doppler ultrasound detected short streaks of blood flow around the hypoechoic areas (Figure 1C,1D). Strain elastography indicated that the mass was softer than the surrounding tissue (Figure 1E,1F). Magnetic resonance imaging (MRI) showed an equal signal in T1-weighted imaging (T1WI) in both breast lesions, a slightly higher signal in T2-weighted imaging (T2WI), and a low signal in the diffusion-weighted imaging (DWI) sequence, with a somewhat rough margin; MRI also indicated double breast-occupying lesions, consistent with BI-RADS 4A, and malignant tumor was diagnosed. After tumor biopsy, a degree of lymphocyte infiltration could be observed around the lobules and stroma of the breast, along with interstitial fibrosis, vitreous degeneration, and fat necrosis with scattered calcification (Figure 2A,2B). After 6 months of follow-up, the patient’s condition was stable. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
SLE, a chronic autoimmune disease that may potentially affect any organ or system, often occurs in women of childbearing age. LP is a rare pathological type of SLE, occurring in 2% to 3% of patients with SLE. Typically, it involves the upper arm, shoulders, face, and buttocks. Meanwhile, LM is a rare manifestation of LP, characterized by inflammation of deep subcutaneous fatty tissue in the breast. The characteristic images include dense lymphoplasmacytic infiltration and transparent fat necrosis in the lobule. LM is more often seen in middle-aged women, can involve both breasts, and has an unclear etiology (2) but only occurs in patients with SLE. Clinical manifestations include single or multiple palpable subcutaneous nodules accompanied by pain, with occasional skin involvement. Other manifestations often overlap with malignant tumor, are susceptible to misdiagnosis, can cause late or improper treatment, and can easily to lead to breast damage; thus, the timely and accurate diagnosis of LP is vital for clinical treatment. Ultrasound is the preferred imaging examination for LM, which is manifests as an irregular hypoechoic area in the mammary gland layer, with an uneven inner echo, unclear boundary, partial lobular shape, coarse or strong arc echo due to fat necrosis, a blood flow signal on color Doppler ultrasound, and lymph node enlargement in the early stage (3). In our case, the ultrasound findings were typical, with an irregular hypoechoic area in the areola region of both breasts, unclear boundaries, and a visibly scattered strong echo. Short strips of blood flow signal were discerned with color Doppler ultrasound. Given the patient’s SLE history, LM was considered, and the diagnosis was accurate. MRI had some utility for LM diagnosis, showing an equal signal in T1WI in both breast lesions, a slightly higher signal in T2WI, and a low signal in the DWI sequence. Pathological confirmation was consistent with LM. LM antimalarial hydroxychloroquine and systemic steroid treatment are effective and can relieve symptoms in 3–6 months. The disease has a tendency to recur, particularly after surgical treatment, so surgical treatment is often deemed unnecessary (4,5).
The diagnosis of LM should be differentiated from inflammatory breast cancer, breast cancer, idiopathic granulomatous mastitis, and subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Inflammatory breast cancer is often accompanied by skin redness, heat, pain, and a palpable large mass with tough texture; ultrasound manifestations are multiple band-like hypoechoic wrapping of fatty tissue, cobblestone-like changes, and visibly dilated lymphatic vessels, with color Doppler showing rich blood flow signal and spectral Doppler showing high speed and high resistance (6). Breast cancer on ultrasound often shows hypoechoic nodules in the gland layer with an aspect ratio greater than 1, irregular morphology, a rough border, crab foot-like changes, rear echo attenuation, and pulling of tissue peripheral to the tumor; under Doppler ultrasound, rich blood flow signals appear to collect in the mass. Idiopathic granulomatous mastitis often occurs in multiparous women, manifesting as a unilateral breast mass, primarily located in the outer upper quadrant, and may be accompanied by pain. For this condition, ultrasonography shows a nonuniform, low-echo region along the catheter with a visible floating spot, while spectral Doppler demonstrates low-resistance blood flow velocity (7). SPTCL is a rare subtype of cutaneous lymphoma characterized by neoplastic T-cell infiltration of subcutaneous tissue, mimicking panniculitis. Ultrasonography of SPTCL typically shows a subcutaneous fat layer with a diffuse strong echo and an unclear boundary. Histopathologically, SPTCL is associated with subcutaneous infiltrates that are similar to those of panniculitis, small- and medium-sized polymorphic T cells, deeply stained nuclei, and an increase to a majority in the number of macrophages. Necrosis, nuclear fragmentation, and phagocytosis are other common manifestations. Immunophenotypically, SPTCL is CD8 positive and CD4 and CD56 negative (8-10).
Conclusions
Ultrasonic panoramic imaging can show the full boundaries and shape of the tumor and determine whether the skin and lymph nodes are involved, Doppler ultrasound can show the blood flow of the lesion, and ultrasound elastography can further evaluate the hardness of the tumor. Combined with a variety of imaging characteristics, these can provide a more accurate basis for determining whether the disease is malignant, and thus multimodal ultrasound provides more valuable information for disease diagnosis. In this case, microflow imaging was not used to evaluate the internal blood flow of the mass, but it can provide a more nuanced picture of blood flow regarding the direction and blood supply vessels.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1279/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roongta R, Joshi S, Chattopadhyay A, Ghosh A. Lupus Mastitis. Reumatol Clin (Engl Ed) 2022;18:312-3. [Crossref] [PubMed]
- Rosa M, Mohammadi A. Lupus mastitis: a review. Ann Diagn Pathol 2013;17:230-3. [Crossref] [PubMed]
- Roongta R, Joshi S, Chattopadhyay A, Ghosh A. Lupus Mastitis. Reumatol Clin (Engl Ed) 2021. [Epub ahead of print]. doi:
10.1016/j.reuma.2021.05.004 . - Oktay A, Esmat HA, Aslan Ö, Mirzafarli I. Lupus Mastitis in a Young Female Mimicking a Breast Carcinoma; a Rare Entity Through a Case Report and Review of the Literature. Eur J Breast Health 2022;18:13-5. [Crossref] [PubMed]
- Summers TA Jr, Lehman MB, Barner R, Royer MC. Lupus mastitis: a clinicopathologic review and addition of a case. Adv Anat Pathol 2009;16:56-61. [Crossref] [PubMed]
- Guo R, Lu G, Qin B, Fei B. Ultrasound Imaging Technologies for Breast Cancer Detection and Management: A Review. Ultrasound Med Biol 2018;44:37-70. [Crossref] [PubMed]
- Nguyen MH, Molland JG, Kennedy S, Gray TJ, Limaye S. Idiopathic granulomatous mastitis: case series and clinical review. Intern Med J 2021;51:1791-7. [Crossref] [PubMed]
- Qiu LH, Tian C. Research Progression of Subcutaneous Panniculitis-like T-Cell Lymphoma--Review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021;29:297-300. [Crossref] [PubMed]
- Baxi KD, Rathod SP, Chaudhary RG, Jagati A. Subcutaneous panniculitis-like T-cell lymphoma. Indian J Dermatol Venereol Leprol 2020;86:606. [Crossref] [PubMed]
- Jeong SI, Lim HS, Choi YR, Kim JW, Park MH, Cho JS, Lee JS, Kang HK. Subcutaneous panniculitis-like T-cell lymphoma of the breast. Korean J Radiol 2013;14:391-4. [Crossref] [PubMed]


