
Rare desmoid-type fibromatosis of the breast in young female patients: a description of three cases and literature analysis
Introduction
Desmoid-type fibromatosis (DF), which falls within the major category of fibroblast and myofibroblast tumors, is classified as intermediate and locally aggressive according to the 2020 World Health Organization (WHO) classification of soft tissue tumors and has an annual incidence of 4–5 cases per million individuals (1-3). Due to the incidence rate of DF in the breast being only 0.2% and its clinical and imaging features partially overlapping with those other breast diseases such as breast cancer, accurate diagnosis is often considerably challenging (4). To the best of our knowledge, there have been limited published cases documenting the diagnosis of DF in the breast thus far. We therefore performed a more comprehensive radiological examination and feature analysis of breast DF as compared to previous studies (5,6).
Case presentation
All procedures performed in this study were in accordance the ethical standards of the Ethics Committee of the West China Hospital of Sichuan University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
Chief complaints
A 29-year-old female was referred to our hospital due to a painful right breast space-occupying lesion.
History of past illness
The patient indicated that she had a history of right breast trauma related to cycling.
Ten years prior to arriving to our center, the patient had inadvertently noticed a small right breast lump without signs of redness, swelling, or pain. One year prior, the patient noticed a gradual increase in the size of her right breast lump and occasional pain. Three months prior, the patient found that the right breast mass was still growing, with an increased frequency of pain.
Physical examination
Bilateral breast asymmetry was observed, characterized by swelling in the upper and outer quadrants of the right breast accompanied by localized skin indentation. No signs of redness, orange peel skin, nipple retraction, or nipple discharge were observed (Figure S1).
Imaging examinations
Ultrasound examinations
On conventional grayscale ultrasound, a huge solid mass (measuring 130 mm × 30 mm × 100 mm size) at the outer quadrant and a relatively small solid mass (measuring 25 mm × 12 mm × 25 mm in size) at the upper inner quadrant were found within the right breast. The aforementioned masses exhibited hypoechoic characteristics, irregular shape, indistinct margins, nonparallel orientation, absence of calcification, posterior echo attenuation, distorted surrounding tissue architecture, infiltration into the subcutaneous layer, and a retro-mammary space [classified as grade 5 in Breast Imaging Reporting and Data System (BI-RADS); Figure 1A]. Doppler flow imaging showed sparse blood flow signals around the masses consistent with Adler grade I (Figure 1B,1C). Additionally, the lesions exhibited a relatively high level of hardness, and shear velocity measurements indicated a median variable speed of 9.69 m/s within the larger lesions (Figure 1D).
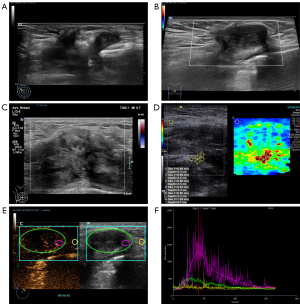
Moreover, the patient underwent a contrast-enhanced ultrasound (CEUS) examination with a bolus injection of 5 mL of contrast agent (SonoVue; Bracco, Milan, Italy) followed by a 3-mL injection of saline through the antecubital vein. Both lesions exhibited heterogeneous enhancement, characterized by a progressive enhancing pattern (Figure 1E,1F).
X-ray and computed tomography (CT) examination
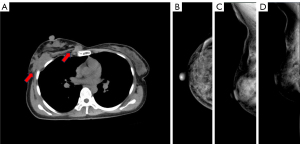
The X-ray revealed an irregular structure, dermal depression, and disordered arrangement within the posterior gap of the right breast. An unenhanced thoracic CT provided a more comprehensive depiction compared to X-ray, revealing the presence of multiple soft tissue masses in the posterior chest wall located behind the right breast, with no bony involvement of underlying ribs (Figure 2).
Magnetic resonance imaging (MRI) examination
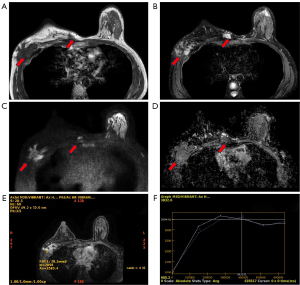
Enhanced breast MRI (Figure 3) was performed. and isointense T1- and hyperintense T2-weighted signal masses were observed in the outer quadrant and upper inner quadrant of the right breast chest wall area, with involvement of the local chest wall muscles; the former extended upwards toward the right axillary tail region and downward to the level of the fifth rib in the pectoralis major muscle area, causing adjacent organizations to deform and exhibiting an indistinct demarcation with breast tissue; meanwhile, the latter extended to the area below the third rib. Moreover, diffusion-weighted imaging (DWI) of the lesions demonstrated a high signal, with the apparent diffusion coefficient (ADC) value measuring 2.42. The two lesions both exhibited heterogeneous enhancement.
Pathology results
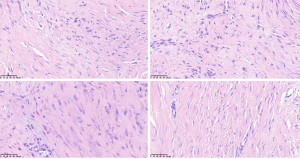
An ultrasound-guided core needle biopsy was carried out to obtain tissues from the masses. The histopathology result showed that the masses were spindle cell proliferative disease. Further immunohistochemistry and genetic testing were performed.
Immunohistochemistry revealed partially nuclear smooth muscle actin (SMA) positivity, scattered nuclear β-catenin positivity, and scattered nuclear desmin positivity in spindle cells. Meanwhile, genetic testing identified a missense mutation in the CTNNB1 gene, which is associated with β-catenin. No mutations were identified in the APC gene, which is associated with familial adenomatous polyposis (FAP). Finally, the masses were confirmed to be DF (Figure 4).
Treatment intervention
A multidisciplinary diagnosis and treatment meeting was held to discuss the therapeutic options for this case. Given the patient’ s younger age and the large size of the masses, it was believed that extensive resection of chest wall mass and muscles would affect organismal function. It was thus decided to decrease the size of the masses so as to reduce risk of surgery trauma and recurrence.
Case 2
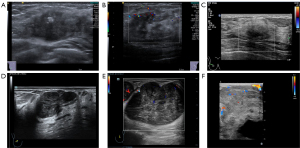
A 30-year-old woman had been experiencing a palpable lump in her right breast for over a year. Ultrasonography revealed a mass measuring 103 mm × 81 mm × 31 mm in size in the right breast. The mass exhibited hypoechoic characteristics, irregular shape, indistinct margins, nonparallel orientation, calcification, posterior echo attenuation, distorted surrounding tissue architecture, infiltration into the subcutaneous layer and retromammary space consistent with Adler grade I. The median variable speed was 9.68 m/s, and there was heterogeneous enhancement on CEUS (BI-RADS 5; Figure 5A-5C). The CT scan revealed the presence of a soft tissue mass in the right breast, while the MRI demonstrated a right breast mass involving the chest wall accompanied by nipple retraction. Pathology confirmed that the mass was DF.
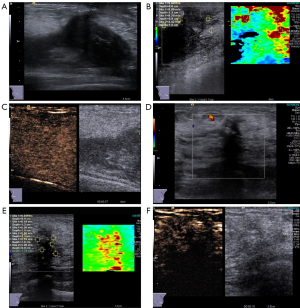
Case 3
A 40-year-old woman indicated the presence for over a month of an inconspicuously palpated mass in her left breast. Ultrasound examination revealed a hypoechoic mass measuring 26 mm × 18 mm × 21 mm in size located at the 4 o’clock position, approximately 2 cm from the nipple. The mass exhibited irregular shape and blurred boundaries with unparallel orientation. No calcifications were observed within the mass, but the posterior echogenicity was attenuated. Surrounding tissue structure remained changed with signs of infiltration (BI-RADS 5). Elasticity analysis indicated a mean variable speed of 5.61 m/s for the mass. Adler grade I and heterogeneous enhancement were observed in the lesion (Figure 5D-5F). X-ray findings showed structural distortion in the outer quadrant of the left breast. DF was ultimately confirmed by pathology.
Discussion
Comprehensive overview of pathological conditions
The 2020 WHO Classification of Soft Tissue Tumors (fifth edition) encompasses 11 primary categories and 176 subtypes, with DF being listed as a rare, locally aggressive, nonmetastasizing mesenchymal tumor. The pathogenesis of DF is associated with genetic, endocrine, and traumatic factors. DF can be classified into three types: extra-abdominal, abdominal wall, and intra-abdominal. Extra-abdominal DF (EADF), which is more commonly observed in adolescent patients, tends to present in the extremities, chest wall/paraspinal region, head, and neck. Abdominal wall DF (AWDF) has a high prevalence among women in their reproductive years and exhibits a greater incidence within the rectus abdominis muscle. Intra-abdominal DF (IADF) is a rare condition that is often associated with FAP/Gardner syndrome. Superficial DF may be amenable to surgical excision without compromising functionality. The National Comprehensive Cancer Network (NCCN) and Desmoid Tumor Working Group guidelines recommend active surveillance or systemic therapy for tumors that are not amenable to surgical resection, such as DF located in the mesentery or neck and adjacent to critical structures (2,7-15).
Distinguishing between DF and breast lesions
This disease typically affects patients at a young age and exhibits a high likelihood of recurrence following surgery; thus, imaging serves as the preferred modality for both the staging of DF and postoperative surveillance. Ultrasound is a convenient imaging modality, lacks ionizing radiation, and is particularly valuable in assessing DF in superficial diseases, such as with AWDF and EADF (5,16,17). DF presents as a fusiform or lobulated mass growing along muscle fibers and fascia, exhibiting sparse blood flow, infrequent liquefaction necrosis, and calcification. In addition, the literature includes two documented characteristic ultrasound features of DF: linear extension of lesions along the fascial plane—referred to as the fascial tail sign—and finger-like extension of lesions into the muscle—known as the staghorn sign (18). The degree of DF reinforcement is correlated with the ratio of fibroblasts to collagen fibers arranged in a shuttle shape, exhibiting a significant augmentation in the densely populated fibroblast region compared to the collagen-rich area (19).
The differential diagnosis of the cases described in this report encompassed considerations of breast cancer, mastitis, sclerosing adenosis, fibroadenoma, borderline phyllodes tumor, and soft tissue sarcoma of the chest (Table 1, Figure 6); the differential features of these conditions are described below: (I) the differential features of breast cancer are microcalcification and heterogeneous hyperenhancement on CEUS with crab foot infiltration or radiation sign. (II) Mastitis usually requires a combination of whether the patient is breastfeeding and has a history of redness, swelling, heat, and pain. The lesion is rich in blood flow and CEUS shows an enlarged lesion. (III) Sclerosing adenosis is categorized as an advanced stage of breast hyperplasia, predominantly observed in middle-aged and older adult females. Ultrasound examination demonstrates a notable posterior acoustic attenuation of the lesion, while imaging reveals low enhancement and limited field of view on CEUS. (IV) Fibroadenomas are typically well-defined and slightly lobulated, exhibiting limited and homogeneous enhancement on CEUS. (V) Borderline phyllodes tumors are characterized by rapid growth within a short duration accompanied by prominent enhancement and demonstrate a pattern of swift progression followed by gradual regression on CEUS. (VI) Soft tissue sarcoma, such as rhabdomyosarcoma, frequently manifests as liquefied necrosis and calcification and is accompanied by abundant blood flow. Imaging reveals heterogeneous hyperenhancement on CEUS.
Table 1
| Entities | Details |
|---|---|
| Desmoid-type fibromatosis | A firm mass, closely associated with the muscular layer, lacking calcification or liquefaction, and demonstrating a progressive enhancement followed by gradual fading on CEUS |
| Breast cancer | A mass exhibiting microcalcification and heterogeneous hyperenhancement and crab foot infiltration or radiation sign on CEUS |
| Mastitis | A lesion with an expanding range and evident vascular or ring enhancement in the surrounding area on CEUS, which may exhibit an internal filling defect when accompanied by an abscess |
| Sclerosing adenosis | A mass with notable reduction in posterior echo attenuation and low enhancement and characterized by a limited field of view on CEUS |
| Fibroadenoma | A well-defined, slightly lobulated mass demonstrating homogeneous enhancement with a limited extent on CEUS |
| Borderline phyllodes tumor | A mass characterized by prominent hyperenhancement followed by gradual washout on CEUS |
| Soft tissue sarcoma | A mass often exhibiting liquefied necrosis and calcification, with heterogeneous hyperenhancement on CEUS |
CEUS, contrast-enhanced ultrasound.
In addition, it is imperative to distinguish EADF and AWDF from soft tissue sarcoma, nodular fasciitis, intramuscular vascular malformation, neurofibroma, and endometriosis (Table 2).
Table 2
| Entities | Details |
|---|---|
| EADF and AWDF | A firm mass, closely associated with the muscular layer, lacking calcification or liquefaction, and demonstrating a progressive enhancement followed by gradual fading on CEUS |
| Soft tissue sarcoma | A mass often exhibiting liquefied necrosis and calcification, with heterogeneous hyperenhancement on CEUS |
| Nodular fasciitis | A mass predominantly observed in the upper extremities and presenting as a rapidly proliferating tender nodule |
| Intramuscular hemangioma | A vascular network with enhanced blood flow upon compression |
| Neurofibroma | An eccentrically expanding neoplasm along the nerves, characterized by a spindle-shaped appearance, rat tail sign, and encapsulation |
| Endometriosis | Purplish-brown lesions at the site of a cesarean section incision, accompanied by cyclic pain associated with menstruation |
EADF, extra-abdominal desmoid-type fibromatosis; AWDF, abdominal wall desmoid-type fibromatosis; CEUS, contrast-enhanced ultrasound.
Additional imaging characteristics of DF
MRI is the preferred modality for the preoperative assessment and postoperative surveillance of AWDF and EADF, while CT is more suitable for assessing IADF. Both primary and recurrent DF often appears as masses with a fascicular configuration and heterogeneous contrast enhancement on MRI. Apart from fascia tail sign on ultrasound, MRI further demonstrates the following characteristics: (I) band sign, presenting as an enhanced mass accompanied by a linear low signal intensity band; (II) separation fat sign, indicating the presence of strip-shaped fat signal between the tumor and muscle; and (III) flame sign, characterized by lesions with feather-like edges resembling flames and equivalent to staghorn sign in ultrasound. It is worth mentioning that an increase in T2 signal intensity on MRI imaging indicates a rise in cell density, thus suggesting an elevated level of invasiveness of the tumor (7,20-23).
This case serves as a reminder that breast lumps may not originate solely from the breast tissue. The presence of tumors with infiltrative growth in the breast should not be immediately diagnosed as breast cancer, and accurate diagnosis of disease requires considering imaging features in combination with clinical history. In case 1, X-ray and CT only indicated disorder in the retromammary space of the breast, while MRI enhancement only suggested the presence of tumor lesions due to an insufficiently elaborated medical history. However, through the integration of ultrasound features and given the clinical history of trauma to the right chest wall, we successfully arrived at a diagnosis of DF. The indispensable advantage of ultrasound examination lies in the ability to effectively communicate with patients and gather comprehensive clinical history during the process of ultrasound examination.
Conclusions
DF is frequently observed in young adults aged 20 to 40 years and is often associated with a history of surgery/trauma or Gardner syndrome. Ultrasound imaging shows DF as a spindle-shaped or lobulated mass in close relation to the muscle layer, demonstrating sparse blood flow and frequently lacking liquefaction, necrosis, and calcification. DF usually appears as fascicular configuration on MRI, which can accurately confirm the extent of muscle involvement. Meanwhile, CT can assess bone and periosteum destruction in patients with DF.
Acknowledgments
The authors thank Dr. Shuang Long, Department of Radiology, Gaoping District People’s Hospital, Nanchong City, and Dr. Yong Cheng, Department of Radiology, West China Hospital of Sichuan University, for providing guidance on radiology knowledge for this article.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1586/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance the ethical standards of the Ethics Committee of the West China Hospital of Sichuan University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Penel N, Bonvalot S, Le Deley MC, Italiano A, Tlemsani C, Pannier D, Leguillette C, Kurtz JE, Toulmonde M, Thery J, Orbach D, Dubray-Longeras P, Verret B, Bertucci F, Guillemet C, Laroche L, Dufresne A, Blay JY, Le Cesne A. Pain in desmoid-type fibromatosis: Prevalence, determinants and prognosis value. Int J Cancer 2023;153:407-16. [Crossref] [PubMed]
- Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, Hohenberger P, Penel N, Messiou C, van der Graaf WT, Gronchi ADesmoid Working Group. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol 2017;28:2399-408. [Crossref] [PubMed]
- Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol 2021;28:44-58. [Crossref] [PubMed]
- Cuomo P, Scoccianti G, Schiavo A, Tortolini V, Wigley C, Muratori F, Matera D, Kukushkina M, Funovics PT, Lingitz MT, Windhager R, Dijkstra S, Jasper J, Müller DA, Kaiser D, Perlaky T, Leithner A, Smolle MA, Campanacci DA. Extra-abdominal desmoid tumor fibromatosis: a multicenter EMSOS study. BMC Cancer 2021;21:437. [Crossref] [PubMed]
- Hennuy C, Defrère P, Maweja S, Thiry A, Gennigens C. Bilateral breast desmoid-type fibromatosis, case report and literature review. Gland Surg 2022;11:1832-41. [Crossref] [PubMed]
- Benej R, Mečiarová I, Pohlodek K. Desmoid-type fibromatosis of the breast: A report of 2 cases. Oncol Lett 2017;14:1433-8. [Crossref] [PubMed]
- Braschi-Amirfarzan M, Keraliya AR, Krajewski KM, Tirumani SH, Shinagare AB, Hornick JL, Baldini EH, George S, Ramaiya NH, Jagannathan JP. Role of Imaging in Management of Desmoid-type Fibromatosis: A Primer for Radiologists. Radiographics 2016;36:767-82. [Crossref] [PubMed]
- Riedel RF, Agulnik M. Evolving strategies for management of desmoid tumor. Cancer 2022;128:3027-40. [Crossref] [PubMed]
- Debaudringhien M, Blay JY, Bimbai AM, Bonvalot S, Italiano A, Rousset-Jablonski C, Corradini N, Piperno-Neumann S, Chevreau C, Kurtz JE, Guillemet C, Bompas E, Collard O, Salas S, Le Cesne A, Orbach D, Thery J, Le Deley MC, Mir O, Penel N. Association between recent pregnancy or hormonal contraceptive exposure and outcome of desmoid-type fibromatosis. ESMO Open 2022;7:100578. [Crossref] [PubMed]
- Mullen JT, Delaney TF, Kobayashi WK, Szymonifka J, Yeap BY, Chen YL, Rosenberg AE, Harmon DC, Choy E, Yoon SS, Raskin KA, Petur Nielsen G, Hornicek FJ. Desmoid tumor: analysis of prognostic factors and outcomes in a surgical series. Ann Surg Oncol 2012;19:4028-35. [Crossref] [PubMed]
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, Dekkers OM, Hogendoorn PC, Vasen HF. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 2011;129:256-61. [Crossref] [PubMed]
- von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J Natl Compr Canc Netw 2020;18:1604-12. [Crossref] [PubMed]
- Rosa F, Martinetti C, Piscopo F, Buccicardi D, Schettini D, Neumaier CE, Gandolfo N, Grazioli L, Gastaldo A. Multimodality imaging features of desmoid tumors: a head-to-toe spectrum. Insights Imaging 2020;11:103. [Crossref] [PubMed]
- Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist 2011;16:682-93. [Crossref] [PubMed]
- The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer 2020;127:96-107. [Crossref] [PubMed]
- Lou L, Teng J, Qi H, Ban Y. Sonographic appearances of desmoid tumors. J Ultrasound Med 2014;33:1519-25. [Crossref] [PubMed]
- Ebrahim L, Parry J, Taylor DB. Fibromatosis of the breast: a pictorial review of the imaging and histopathology findings. Clin Radiol 2014;69:1077-83. [Crossref] [PubMed]
- Garcia-Ortega DY, Martín-Tellez KS, Cuellar-Hubbe M, Martínez-Said H, Álvarez-Cano A, Brener-Chaoul M, Alegría-Baños JA, Martínez-Tlahuel JL. Desmoid-Type Fibromatosis. Cancers (Basel) 2020;12:1851. [Crossref] [PubMed]
- Rhim JH, Kim JH, Moon KC, Park SW, Sohn CH, Choi SH, Yun TJ, Chang KH. Desmoid-type fibromatosis in the head and neck: CT and MR imaging characteristics. Neuroradiology 2013;55:351-9. [Crossref] [PubMed]
- Gondim Teixeira PA, Biouichi H, Abou Arab W, Rios M, Sirveaux F, Hossu G, Blum A. Evidence-based MR imaging follow-up strategy for desmoid-type fibromatosis. Eur Radiol 2020;30:895-902. [Crossref] [PubMed]
- Timbergen MJM, Starmans MPA, Padmos GA, Grünhagen DJ, van Leenders GJLH, Hanff DF, Verhoef C, Niessen WJ, Sleijfer S, Klein S, Visser JJ. Differential diagnosis and mutation stratification of desmoid-type fibromatosis on MRI using radiomics. Eur J Radiol 2020;131:109266. [Crossref] [PubMed]
- Sedaghat S, Sedaghat M, Krohn S, Jansen O, Freund K, Streitbürger A, Reichardt B. Long-term diagnostic value of MRI in detecting recurrent aggressive fibromatosis at two multidisciplinary sarcoma centers. Eur J Radiol 2021;134:109406. [Crossref] [PubMed]
- Sedaghat S, Surov A, Krohn S, Sedaghat M, Reichardt B, Nicolas V. Configuration of Primary and Recurrent Aggressive Fibromatosis on Contrast-Enhanced MRI with an Evaluation of Potential Risk Factors for Recurrences in MRI Follow-Up. Rofo 2020;192:448-57. [Crossref] [PubMed]


