
Bacteremia caused by Bartonella in a 48-year-old woman with a large lung mass
Introduction
Bartonella species have been discovered in a wide range of mammals, with the related DNA being be found in multiple vectors. Bacteremia within a diverse array of reservoirs hosts is a typical manifestation of infection by Bartonella spp. Bartonella spp. bacteria are the causative agents of multiple human diseases. The most well-known infection is cat scratch disease (CSD), which is characterized by lymphadenitis, can also involve other systems, and may result in major illness and a fever of unknown origin (1-3). Although many systemic manifestations of CSD have been described, pulmonary involvement has rarely been reported (4,5). The purpose of this case report is to describe a unique clinical course of CSD in a patient who presented with a pulmonary mass, lymphadenitis, and liver damage. Of note, malignancy or lymphoma was the first radiological interpretation of the lung lesion in our patient, but further investigations revealed the presence of Bartonella genus DNA.
Case presentation
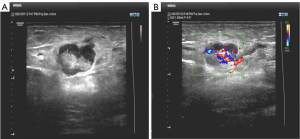
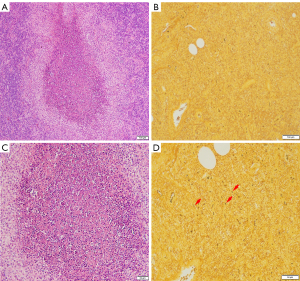
A 48-year-old woman was admitted to Binzhou Medical University Hospital with a 20-day history of increasing pain and left arm swelling. Her left supratrochlear lymph node was enlarged and tender. She had no other constitutional symptoms. Ultrasonograms of the left upper arm showed not well-defined, solid masses with prominent vascular pedicles—typical characteristics of enlarged lymph nodes (Figure 1). Her medical history was unremarkable: she was immunocompetent, had no other coexisting diseases, and had not travelled abroad. Although she had been in close contact with dogs, she did not remember having any dog or cat scratches. A biopsy of the supratrochlear lymph nodes was performed by a surgeon, and pathological analysis revealed necrotizing granulomatous infiltration that was consistent with CSD. Warthin Starry staining showed aggregates of rod-shaped bacteria in the tissue (Figure 2). Accordingly, the patient was treated with oral azithromycin (10 mg/kg, once daily) for 7 days. The pain and swelling of her elbow progressively resolved. However, the patient experienced a high fever (39.0 °C) and dry cough approximately 6 weeks later and was referred to our hospital for further investigation.
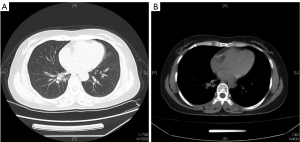
Computed tomography (CT) of the chest and abdomen performed after the administration of intravenous contrast revealed a rounded mass in the right lower lobe near the hilum, and the mass measured approximately 5.0 cm × 6.0 cm in size. Lymph nodes in the mediastinum and right hilum were abnormally enlarged, extended around the esophagus, and were not clearly demarcated from the esophagus (Figure 3). These findings were most consistent with central bronchial carcinoma, but pneumonia could not be excluded. The liver and spleen were normal. Laboratory tests revealed a white blood cell count of 9,900/µL (neutrophils, 81.3%; lymphocytes, 10.6%; monocytes, 6.9%), a hemoglobin level of 12.0 g/dL, a platelet count of 338,000/µL, a C-reactive protein level of 30.1 mg/L, and a procalcitonin level of 0.05 ng/mL. Serum biochemical tests suggested no impairment of liver function. Tests for coronavirus disease 2019 (COVID-19), tuberculosis, and HIV were negative. Multiple blood cultures were sterile. An autoimmune workup including antinuclear antibodies, rheumatoid factor, and antineutrophil cytoplasmic antibodies were also negative.
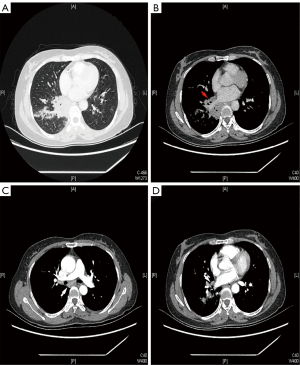
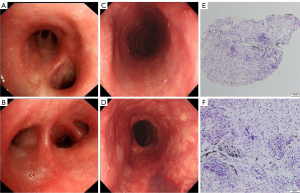
The patient underwent bronchoscopy, which showed that all the bronchi were unobstructed and that there was only mild edema of the mucosa (Figure 4A,4B). The endoscopist performed a biopsy, alveolar lavage, and brush examination. Meanwhile, gastroduodenoscopy showed that the esophageal mucosa was smooth (Figure 4C,4D). Pathological analysis of bronchoscopy biopsy tissue and bronchoalveolar lavage failed to show any evidence of pathogens or malignancy (Figure 4E,4F). Moxifloxacin hydrochloride (0.4 g) was administered intravenously for 14 days. The patient’s temperature gradually decreased to normal levels, her cough was relieved, and she was discharged. However, the patient experienced fatigue and abdominal distension 4 weeks postdischarge but did not experience fever during this period. A liver function test revealed that alanine aminotransferase (ALT) levels were 120.8 U/L and that aspartate aminotransferase (AST) levels were 96.7 U/L. We performed chest and upper abdomen CT examination. The upper abdomen CT revealed no liver abnormalities. The mass in the lower lobe of the right lung was significantly smaller than previously. Considering the above examination results, we diagnosed the mass as inflamed lung tissue. To identify the pathogen associated with pneumonia, a polymerase chain reaction (PCR) test was performed by Guangzhou KingMed Diagnostics Group. Extraction of the DNA from the whole-blood sample and amplification of Bartonella genus DNA by use of PCR methods (with primer 5'-3': forward TGCTCATAGACGTCAATGCC; reverse GCTACGGCCCCTAAATCAG) were performed, and the result was positive. A multidisciplinary treatment plan was organized for the subsequent treatment measures. After discussion, we concluded that the pneumonia and lymphadenopathy were related to a Bartonella infection. Ultrasound-guided puncture of the lung mass was recommended to define the nature of the mass, but the patient refused. Oral azithromycin treatment (500 mg/day) was administered for 6 weeks. Two months later, a liver function test indicated ALT levels of 39.3 U/L and AST levels of 44.0 U/L. The patient was reexamined with chest CT, which showed that the mass and abnormally enlarged lymph nodes had almost disappeared (Figure 5). The patient did not experience any discomfort at the 2-month follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion and conclusions
Bartonella infections have gained considerable attention in international public health over the past few decades. These bacteria cause a growing clinical spectrum, which often results in a challenge for clinicians due to the limitations of the microbiological tests used for diagnosis. Although a history of exposure to cats is important, infection of humans following contact with dogs has also been reported. Because dogs are fully susceptible to several zoonotic Bartonella species and likely incidental hosts of Bartonella henselae (6). Most patients with CSD have a benign, self-limiting clinical course characterized by isolated lymphadenopathy with fever and no other signs or symptoms. However, up to 25% of infected patients can develop hepatic and/or splenic granulomas or atypical manifestations (7-9) Although the disease may involve multiple systems, there are only a few cases of lung involvement. Marseglia et al. (10) reported a child patient with CSD involving the lungs with lung CT showing diffuse nodular infiltrates in both lung fields. Black et al. (11) reported a case of life-threatening CSD in an immunocompromised host whose chest roentgenogram demonstrated a widened mediastinum and bilateral infiltrates, which was consistent with a diffuse infectious process or adult respiratory distress syndrome. In our case, chest CT revealed a large mass and abnormally enlarged lymph nodes within the mediastinum. This led to the first radiological interpretation being a malignancy or lymphoma. Pulmonary CSD is relatively rare clinically, and this case demonstrates how the diagnosis of atypical CSD can be challenging. The nonspecific, composite, and variable clinical features of CSD require careful evaluation to achieve a precise diagnosis and to avoid a delayed diagnosis (12). Another characteristic of this case was that the patient’s liver function was impaired. This could have been related to CSD, and granulomatous hepatitis has been reported in association with B. henselae in dogs, horses, and human patients. The typical symptoms include right upper quadrant pain, fever, and weight loss. Histologically, hepatic bartonellosis is characterized by the presence of necrotizing granulomas, but the diagnosis and therapy of hepatic bartonellosis is exceedingly difficult (13,14). The liver function damage could have also been related to medication. Fortunately, she was completely cured.
Bartonella infections in immunocompetent individuals usually have self-limited clinical courses with a gradual yet total resolution of lymphadenopathy within 2–6 months (15). If the disease involves other systems, antibiotics may be helpful; however, antibiotic selection and the period of antibiotic use have long been controversial. Antibiotics that have been used to treat patients with CSD include azithromycin, rifampin, ciprofloxacin, and gentamicin (16,17). The duration of antibiotic treatment may range from 4 days to 4 months (7), depending on the severity of the illness. Since lung-involved CSD is highly rare in immunocompetent patients, the duration of therapy and antibiotic choices are not well established. The patient described here received oral azithromycin for 6 weeks, which resulted in a good response and positive clinical outcome. She had no fever, and the mass and abnormally enlarged lymph nodes had almost completely disappeared.
In conclusion, Bartonella is able to infect almost any organ in humans, and the clinical manifestation of CSD can vary. Although lung damage associated with CSD is rare in immunocompetent patients, Bartonella infection may manifest as a large lung mass in immunocompetent patients, as in our case. Serology is the best initial test and can be performed with indirect fluorescence or enzyme-linked immunosorbent assay. PCR can be used to detect and identify different Bartonella species. Antibiotic treatment is considered effective, but patients may need to stay on the medication for 6 weeks. Consequently, when a patient presents with lymphadenopathy, any history of cat or dog exposure should be queried, and appropriate tests should be ordered.
There are certain limitations of our case that should be mentioned. Serology is often recommended as a useful test for the diagnosis of CSD and evaluating treatment success in patients with enduring bacteremia; however, it was not performed in our case. Going forward, we will be more thorough and rigorous in cases of this kind on clinical visits.
Acknowledgments
We thank Guangzhou KingMed Diagnostics Group for providing information of PCR conditions, primers, and the English version of the report.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1390/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheslock MA, Embers ME. Human Bartonellosis: An Underappreciated Public Health Problem? Trop Med Infect Dis 2019;4:69. [Crossref] [PubMed]
- Maggi RG, Halls V, Krämer F, Lappin M, Pennisi MG, Peregrine AS, et al. Vector-borne and other pathogens of potential relevance disseminated by relocated cats. Parasit Vectors 2022;15:415. [Crossref] [PubMed]
- Landes M, Maor Y, Mercer D, Habot-Wilner Z, Bilavsky E, Chazan B, et al. Cat Scratch Disease Presenting as Fever of Unknown Origin Is a Unique Clinical Syndrome. Clin Infect Dis 2020;71:2818-24. [Crossref] [PubMed]
- Nawrocki CC, Max RJ, Marzec NS, Nelson CA. Atypical Manifestations of Cat-Scratch Disease, United States, 2005-2014. Emerg Infect Dis 2020;26:1438-46. [Crossref] [PubMed]
- Luciani L, El Baroudi Y, Prudent E, Raoult D, Fournier PE. Bartonella infections diagnosed in the French reference center, 2014-2019, and focus on infections in the immunocompromised. Eur J Clin Microbiol Infect Dis 2021;40:2407-10. [Crossref] [PubMed]
- Chomel BB, Ermel RW, Kasten RW, Henn JB, Fleischman DA, Chang CC. Experimental infection of dogs with various Bartonella species or subspecies isolated from their natural reservoir. Vet Microbiol 2014;168:169-76. [Crossref] [PubMed]
- Klotz SA, Ianas V, Elliott SP. Cat-scratch Disease. Am Fam Physician 2011;83:152-5. [PubMed]
- Minadakis G, Angelakis E, Chochlakis D, Tselentis Y, Psaroulaki A. Cat-scratch disease in Crete: an update. Infect Dis Rep 2011;3:e15. [Crossref] [PubMed]
- Rodríguez C M, Giachetto L G, Cuneo E A, Gutiérrez B, Mdel C, Shimchack R M, Pírez G MC. Cat-scratch disease with bone compromise: atypical manifestation. Rev Chilena Infectol 2009;26:363-9. [PubMed]
- Marseglia GL, Monafo V, Marone P, Meloni F, Martini A, Burgio GR. Asymptomatic persistent pulmonary infiltrates in an immunocompetent boy with cat-scratch disease. Eur J Pediatr 2001;160:260-1. [Crossref] [PubMed]
- Black JR, Herrington DA, Hadfield TL, Wear DJ, Margileth AM, Shigekawa B. Life-threatening cat-scratch disease in an immunocompromised host. Arch Intern Med 1986;146:394-6. [Crossref] [PubMed]
- Sodini C, Zani EM, Pecora F, Conte C, Patianna VD, Prezioso G, Principi N, Esposito S. A Case of Atypical Bartonellosis in a 4-Year-Old Immunocompetent Child. Microorganisms 2021;9:950. [Crossref] [PubMed]
- Álvarez-Fernández A, Breitschwerdt EB, Solano-Gallego L. Bartonella infections in cats and dogs including zoonotic aspects. Parasit Vectors 2018;11:624. [Crossref] [PubMed]
- VanderHeyden TR, Yong SL, Breitschwerdt EB, Maggi RG, Mihalik AR, Parada JP, Fimmel CJ. Granulomatous hepatitis due to Bartonella henselae infection in an immunocompetent patient. BMC Infect Dis 2012;12:17. [Crossref] [PubMed]
- Eleftheriotis G, Skopelitis E. Concurrence of cat-scratch disease and paradoxical tuberculosis-IRIS lymphadenopathy: a case report. BMC Infect Dis 2022;22:213. [Crossref] [PubMed]
- Bass JW, Freitas BC, Freitas AD, Sisler CL, Chan DS, Vincent JM, Person DA, Claybaugh JR, Wittler RR, Weisse ME, Regnery RL, Slater LN. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J 1998;17:447-52. [Crossref] [PubMed]
- Margileth AM. Antibiotic therapy for cat-scratch disease: clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr Infect Dis J 1992;11:474-8. [Crossref] [PubMed]


