Factors influencing the accuracy and safety of preoperative computed tomography (CT)-guided soft hook-wire localization for pulmonary nodules: a comprehensive analysis
Introduction
Lung cancer is a major contributor to cancer-related deaths globally, with an overall 5-year survival rate of 29.7%; however, early diagnosis allows an increase in the survival rate of up to 82% (1). Therefore, early detection of lung cancer, followed by timely surgery, is crucial for improving patient survival rates. Lobectomy is currently considered the most effective surgical treatment for early lung cancer. Further, a recent meta-analysis indicated that the long-term survival outcomes of lobectomy are comparable to those of extended segmentectomy for peripheral non-small cell lung cancer (NSCLC) (2-4). Nevertheless, computed tomography (CT)-guided preoperative positioning of lung nodules can be beneficial to ensure adequate margins and reduce the time required for surgery in patients undergoing segmentectomy (5).
Video-assisted thoracoscopic surgery (VATS) has several limitations in identifying and localizing small peripheral pulmonary nodules, particularly deep ground-glass nodules. To address this, preoperative positioning techniques have been developed to ensure accurate intraoperative identification and precise nodule removal. Currently, CT guidance is the most widely used method for percutaneous perthoracic localization. Several CT guidance techniques are currently available, including metal markers such as hooked wires and microcoils, or dye staining (6-10). Further, several methods can be used to achieve preoperative labeling of lung nodules, each with its own advantages and disadvantages. One such method is microcoil localization, which can achieve good accuracy and a high success rate. However, this method is relatively complex and carries the risk of displacement (11-13).
Our team utilizes a novel device comprising a flexible wire claw-hook positioner for preoperative lung nodule positioning. This device has a hook-claw device at the tip and a soft wire at the tail, making it stronger and safer after release. The accuracy of CT-guided localization of lung nodules directly affects the surgical effect and intraoperative risk; therefore, it is necessary to analyze the factors influencing accuracy and post-localization complications (13,14).
Herein, we analyzed the outcomes of 281 patients who underwent resection with a soft wire claw-hook to locate pulmonary nodules, to identify the factors affecting the accuracy of marking and the incidence of complications. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1272/rc).
Methods
This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Nanjing Drum Tower Hospital. Written informed consent was provided by all patients prior to performing the CT-guided percutaneous localizations procedure.
Study participants
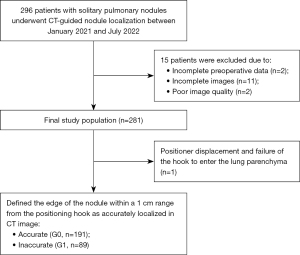
Between January 2021 and July 2022, 281 patients with solitary pulmonary nodules underwent CT-guided nodule localization using a soft hook-wire and subsequent VATS resection in our institute. The decision to request preoperative nodule localization was made at the discretion of the referring thoracic surgeon. CT morphology and location were the primary factors used to identify eligible lesions. Ground-glass opacities that were unlikely to be palpable during surgery, pulmonary nodules smaller than 3 cm in diameter, nodules located less than 3 cm from the nearest pleural surface, biopsy-confirmed malignant nodules, and malignant nodules with suspicious morphological and septal changes were all considered eligible. Preoperative nodule localization was performed in patients who met the following criteria: no history of extrathoracic malignant tumors, VATS resection performed within 24 hours of localization, accurate resection, and clear postoperative pathological results. A total of 2 cases were excluded due to incomplete preoperative data, 11 cases were excluded due to incomplete images, and 2 cases were excluded due to poor image quality caused by difficulties in ensuring breathing coordination (Figure 1).
Demographic information, including sex, age, and smoking history, was recorded. Additionally, a preoperative comprehensive assessment of the heart, lung, and coagulation functions were recommended in patients before VATS (15). Prior to the CT scan, patients were instructed to perform and maintain calm breathing.
Preprocedure image analysis
Preoperative images were acquired using a Spectral CT scanner (IQon Spectral CT, Philips Healthcare, Best, Netherlands) with the following scan parameters: tube current, 50–250 mAs; tube voltage, 120 kV; pitch factor, 1.2–1.5; reconstruction algorithm, Y-Detail; reconstruction slice thickness, 1 mm; interval, 1 mm; and field of view (FOV), 350×350 mm. Indices were measured at the lung window [window width, 1,500 Hounsfield units (HU); window level, 600 HU].
Then, 2 radiologists, one (Q.F.) with 5 years of experience in interventional thoracic radiology and the other (J.Z.) with 8 years of experience in thoracic radiology, reviewed the last diagnostic CT scan obtained prior to CT-guided placement. During the measurement process, researchers remained blind to the patients’ clinical and surgical details, as well as to the pathological outcomes. In case of any disagreement, a radiologist with 14 years of experience (L.C. and W.K.) performed the final review.
Preoperative images were carefully analyzed and recorded to determine the location, size (maximum diameter), and nature (ground-glass, mixed ground-glass, or solid) of the pulmonary nodules. These measurements were performed to better understand the characteristics of the nodules prior to surgery.
CT-guided soft hook-wire localization
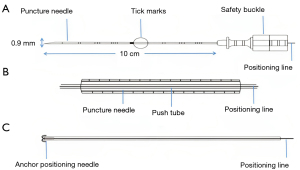
All surgeries were performed with the patient awake and quiet. All CT scans were performed using an IQon Spectral CT Scanner (Philips, Netherlands) at 100 KV, 120 mAs, 1 mm thickness. We evaluated nodule location on the lung window image. We selected the optimal route, and confirmed the position away from the blood vessel along the puncture route. Generally, this method allows selection of the shortest route for needle entry from the skin to a pulmonary nodule. Ideally, the marking position should be 1 cm from the edges of the pulmonary nodules. The positioning line provided by the scanner is used to mark the patient’s body surface. Local subcutaneous infiltration of 3–5 mL of 2% lidocaine was applied as anesthesia. A soft-wire hook puncture needle (20 G × 100 mm; Ningbo Shengjiekang Biotechnology Co., Ltd., Ningbo, China) was used for nodule localization. The structure and positioning principle of the needle are shown in Figure 2. The locating needle comprised 5 main parts: a puncture needle, push device, anchor positioning needle, positioning line, and protection tube. The puncture needle comprised a needle tube, puncture needle handle, and safety buckle (Figure 2A). The push device was composed of a push tube and push tube handle (Figure 2B). The far end of the positioning line was connected to an anchor positioning needle (Figure 2C). The positioning line extended from the proximal end of the push device through the interior and outside of the push tube, and was marked with a tick at the distal end, measuring 10–15 cm in length and 0.9 mm in diameter.
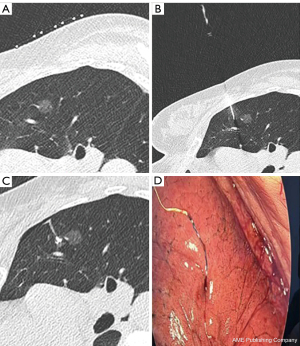
Under CT guidance, a wire hook was advanced into the lung parenchyma surrounding the nodules. Once the positioner was released, the operator removed the positioning needle and bandaged the puncture points. A repeat CT scan was performed immediately to confirm that the soft-line hook had been properly placed in the lung parenchyma. This step is crucial to assess the relative position of the hook claw to the target nodule and pleural surface, as well as to identify any possible complications, such as hemorrhage or pneumothorax (Figure 3).
Postprocedure image analysis
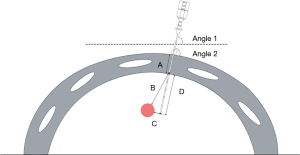
The CT-guided puncture localization-related images were retrospectively analyzed by 2 attending physicians (Q.F. and J.Z.) with 5 and 8 years of experience in radiology. Any disagreements were resolved by the chief physician with 14 years of experience in radiology who reviewed the images again. Based on the CT images, the puncture position (supine, prone, left, right, oblique), side of the nodule (left or right), and location of the pulmonary nodule (upper, middle, or lower lobes) were documented. We measured various parameters related to the pulmonary nodule, including its maximum diameter, the shortest distance from the outer edge of the nodule to the point at which the puncture needle enters the lung (known as the nodule depth), the thickness of the chest wall where the needle was inserted, the depth of the needle in the lung, the angle at which the puncture needle entered the chest wall (angle 1), and the angle between the puncture needle and the horizontal plane (angle 2) (Figure 4).
Surgical resection and pathological outcomes
The decision to perform VATS within 24 hours after positioner placement was made at the discretion of a thoracic surgeon. Surgery was often performed relatively early in patients who were elderly, had difficulty tolerating the condition, or had a massive pneumothorax. During VATS, the thoracic surgeons were able to directly observe the line outside the pleura and manually palpate the focus.
After identifying the localization marks, the thoracic surgeon resected the corresponding lung tissue along with the localization of the claw hooks and specimens. All the resected specimens were promptly sent for histopathological examination. The surgeon decided whether to perform segmentectomy or lobectomy immediately after wedge resection based on the results of the intraoperative frozen section and/or lymph node sampling.
Statistical analysis
In VATS, the surgeon considers the positioning needle technically successful when the tail positioning line of the device can be directly observed after entering the chest cavity. Nodules located within 1 cm of the positioning hook on CT images were classified as accurately positioned (G0), whereas those located outside of this range were classified as inaccurately positioned (G1). All factors that may affect the positioning accuracy were included in the statistical analysis. These included demographic factors, such as age, sex, history of emphysema, and history of extrapulmonary malignancies; nodule factors, including size (maximum diameter), solidity, density, location, and depth; and technical factors, including patient position, interlobar fissure, thoracic wall thickness, needle depth, the angle between the needle and pleura (angle 1), and the angle between the needle and horizontal plane (angle 2). During surgery, we considered that there was an increased risk of puncturing the bronchial artery when the depth of the pulmonary nodule was greater than 2.5 cm. Therefore, depth of the pulmonary nodule beyond 2.5 cm was treated as a separate influencing factor.
Data analysis was performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). Continuous variables, including age, nodule size, and distance from the nodule to the pleura, are presented as means ± standard deviation (SD), with ranges in parentheses. We used 2-sample t-tests to compare continuous variables. Categorical variables, such as sex and nodule location, were presented as counts or counts with percentages in parentheses. Chi-squared and Fisher’s exact tests were used to compare categorical variables. Linear and multivariate logistic regression analyses were performed, using all of the aforementioned variables. Statistical significance was set at P<0.05. We conducted a 2-sided test for the P values.
Results
Demographics of the study cohort
A total of 281 patients (187 women and 94 men) aged 53.5±12.5 years (range, 21–81 years) underwent CT-guided soft wire hook placement. One patient was excluded from the study because of positioner displacement and failure of the hook to enter the lung parenchyma resulting from a pneumothorax. All patients were subsequently followed up using VATS. The characteristics of all patients and pulmonary nodules are listed in Table 1. Postoperative pathological examination revealed that 53 cases (18.9%) were benign and 227 (81.0%) were malignant (Table 2).
Table 1
| Variables | Value |
|---|---|
| Age (year) | 53.5±12.5 |
| Gender (female/male) | 187 (66.5)/94 (33.5) |
| Smoking status (ex-or current/never) | 106 (37.7)/175 (62.3) |
| History of emphysema (yes/no) | 82 (29.2)/199 (70.8) |
| History of extrathoracic malignancy (yes/no) | 5 (1.8)/275 (98.2) |
| Nature (pure GGO/part solid/solid) | 153 (54.4)/108 (38.4)/19 (6.8) |
| Location (upper lobe/middle lobe/lower lobe) | 143 (50.9)/22 (7.8)/115 (40.9) |
| Surgery (VATS wedge resection/segmentectomy/lobectomy/conversion to thoracotomy) | 52 (18.5)/186 (66.2)/43 (15.3)/0 (0) |
Continuous variables are presented as mean ± SD and categorical variables are presented as numbers (%). GGO, ground-glass opacity; VATS, video-assisted thoracoscopic surgery; SD, standard deviation.
Table 2
| Pathologic finding | Value |
|---|---|
| Benign | 53 (18.9) |
| Malignancy | 227 (81.0) |
| Adenocarcinoma in situ | 64 (22.8) |
| Minimally invasive adenocarcinoma | 113 (40.2) |
| Invasive adenocarcinoma | 43 (15.3) |
| Squamous cell carcinoma | 2 (0.71) |
| Metastasis | 5 (1.78) |
Data are shown as counts with percentages in parentheses.
Factors influencing successful localization
During VATS, the location of the hook wire and pulmonary nodules were explored and removed. The CT scan image after localization was used to define the edge of the nodule within a 1 cm range from the positioning hook as accurately localized. Of the 280 patients, 191 (68.2%) were classified as accurate (G0) and 89 (31.7%) were classified as inaccurate (G1).
Univariate analysis revealed that demographic factors and nodule properties such as size and density had no significant impact on accurate nodule localization. However, nodule location, depth, and needle depth were identified as crucial factors influencing precise localization, as shown in Table 3. Further, we found that nodule depth was significantly different between the accurate (1.01 cm) and inaccurate (2.24 cm) groups (P<0.001). Specifically, when the nodule depth was less than 2.5 cm, the localization accuracy was 67.5%; however, when the nodule depth was greater than 2.5 cm, the localization accuracy decreased significantly to only 0.7% (P<0.001). This result indicates that needle depth is a significant factor influencing accurate localization. The average distance in the accurate group was 0.9 cm, whereas the average distance in the inaccurate group was 1.1 cm (P<0.001). Thoracic wall thickness, the angle between the needle and pleura, and the angle between the needle and horizontal plane were not important factors for positioning accuracy. Multivariate regression analysis comprehensively assessed all the important factors (including demographics, nodule factors, and technical factors). Overall, nodule depths [odds ratio (OR) =22.610, 95% confidence interval (CI): 10.351–49.391, P=0.001] and needle depths (OR =0.3 22, 95% CI: 0.136–0.765, P=0.010) were identified independent factors associated with accurate localization (Table 4).
Table 3
| Characteristic | G0 (n=191) | G1 (n=89) | P value |
|---|---|---|---|
| Demographic factors | |||
| Age (years) | 53.9±12.5 | 52.6±12.3 | 0.85 |
| Gender (female/male) | 134 (70.2)/57 (29.8) | 52 (58.4)/37 (41.6) | 0.05 |
| History of emphysema (yes/no) | 62 (32.5)/129 (67.5) | 19 (21.3)/70 (78.7) | 0.05 |
| History of extrathoracic malignancy (yes/no) | 3 (1.6)/188 (68.4) | 2 (2.2)/87 (97.8) | 0.69 |
| Nodule characteristics | |||
| Size (cm) | 1.1±4.6 | 0.9±0.7 | 0.42 |
| Solidity (pure GGO/part solid/solid) | 110 (57.6)/71 (37.2)/10 (5.2) | 43 (48.3)/37 (41.6)/9 (10.1) | 0.18 |
| Density | −434.7±230.1 | −427.1±235.6 | 0.82 |
| Location (specific to the lobes) | 0.16 | ||
| Left upper/left lower | 49 (25.7)/34 (17.8) | 15 (16.9)/13 (14.6) | |
| Right upper/right middle/right lower | 46 (24.1)/14 (7.3)/48 (25.1) | 33 (37.1)/8 (9.0)/20 (22.5) | |
| Location (specific to the segment) | – | – | 0.02 |
| Nodule depth (>2.5/≤2.5 cm) | 189 (99.0)/2 (1.0) | 57 (64.0)/32 (36.0) | <0.001 |
| Technical factors | |||
| Patient position | 0.44 | ||
| Supine/prone/left lateral | 47 (24.6)/65 (34.0)/19 (9.9)/ | 20 (22.5)/33 (37.1)/8 (9.0) | |
| Right lateral/oblique lateral | 17 (8.9)/43 (22.5) | 3 (3.4)/25 (28.1) | |
| Through the interlobar fissure (yes/no) | 188 (98.4)/3 (1.6) | 86 (96.6)/3 (3.4) | 0.33 |
| Nodule depth (cm) | 1.01±0.5 | 2.24±0.8 | <0.001 |
| Thoracic wall thickness (cm) | 3.8±1.1 | 4.2±1.2 | 0.71 |
| Needle depth (cm) | 0.9±0.3 | 1.1±0.5 | <0.001 |
| Angle between needle and pleura (angle 1) | 75.9±9.2 | 75.3± 8.7 | 0.57 |
| Angle between needle and horizontal plane (angle 2) | 73.8±13.8 | 72.4±15.3 | 0.48 |
Continuous variables are presented as mean ± SD and categorical variables are presented as numbers (%). Nodule depth, distance from nodule to the pleura. Needle depth, distance from needle tip to the pleura. G0: the edge of the pulmonary nodule was less than 1 cm from the localization needle tip on CT. G1: the edge of the pulmonary nodule was more than 1 cm from the localization needle tip on CT. GGO, ground-glass opacity; SD, standard deviation; CT, computed tomography.
Table 4
| Variable | β value | OR | 95% CI | P value |
|---|---|---|---|---|
| Size (cm) | −0.030 | 0.966 | 0.866–1.076 | 0.526 |
| Solidity | −0.010 | 0.999 | 0.997–1.001 | 0.180 |
| Location | 0.098 | 1.103 | 0.863–1.410 | 0.433 |
| Patient position | 0.066 | 1.068 | 0.837–1.364 | 0.595 |
| Nodule depth (cm) | 3.118 | 22.610 | 10.351–49.391 | 0.001 |
| Needle depth (cm) | −1.132 | 0.322 | 0.136–0.765 | 0.010 |
| Thoracic wall thickness (cm) | 0.240 | 1.271 | 0.928–1.741 | 0.135 |
| Angle between needle and pleura (angle 1) | −0.190 | 0.982 | 0.942–1.023 | 0.377 |
| Angle between needle and horizontal plane (angle 2) | −0.160 | 0.984 | 0.959–1.010 | 0.225 |
OR, odds ratio; CI, confidence interval.
Factors influencing the risk of complications
Of the 281 patients, 64 (22.8%) experienced self-limiting bleeding, such as needle-tract bleeding and hemoptysis. Fortunately, none of these cases escalated to the point where thoracotomy was required (Table 5). According to the results of the univariate analysis, several factors, including nodule diameter, nodule depth, chest wall thickness, and needle depth, showed a significant causative link with bleeding. The mean nodule diameter in the bleeding group was 1.99 cm, which was significantly higher than the mean nodule diameter of 0.84 cm in the non-bleeding group (P=0.036). The mean nodule depth was 1.64 cm in the bleeding group and 1.33 cm in the non-bleeding group (P=0.011). Further, the mean chest wall thickness was 3.91 cm in the non-bleeding group and 4.26 cm in the bleeding group (P=0.043). The mean needle insertion depth was 1.01 cm in the non-bleeding group and 1.18 cm in the bleeding group (P=0.005).
Table 5
| Characteristic | Value |
|---|---|
| Pneumothorax (self-limiting/requiring chest tube or immediate surgery) | 84 (29.9)/5 (1.8) |
| Hemorrhage or hemoptysis positioner displaced or dislodged | 64 (22.8) |
Data are shown as counts with percentages in parentheses.
Of the 281 patients analyzed, 84 (29.9%) experienced pneumothorax, and 5 had to undergo early surgery. The incidence of pneumothorax was not associated with demographic or nodular factors. Patients with pleural reactions were administered oxygen inhalation and 5% glucose solution infusion, and were closely monitored and treated. No other interventions were administered.
Discussion
Our study comprehensively analyzed the factors affecting the accuracy of pulmonary nodule localization before VATS, and discussed the influencing factors of complications.
VATS has recently emerged as the preferred surgical approach for the diagnosis and treatment of early lung cancer (16). Surgeons often face challenges in accessing and visualizing pulmonary nodules located deep within the lung parenchyma, as well as for small ground-glass nodules. Preoperative localization of these nodules with VATS enables chest surgeons to achieve precise resection, thereby minimizing the need for extensive surgery, and promoting the use of minimally invasive treatments. Consequently, it is crucial to assess the factors that affect the success rate, safety, and risks associated with the preoperative localization of pulmonary nodules.
In the present study, successful localization was defined as having the locator positioned within the lung parenchyma, with the tail end of the cord visible on the skin surface or outside the lung pleura, and directly visible to the chest surgeon during surgery. To evaluate the accuracy of localization, we measured the shortest distance (1 cm) between the release position of the needle tip and the edge of the pulmonary nodule. This standard fully satisfies the requirements of VATS for lesion localization. During VATS, all localization markers, including the pulmonary nodules, were removed and subsequently verified by pathological examination.
In our study, only 1 patient experienced claw dislodgement during repeated scans, whereas the remaining 280 positioned needles were successfully arranged. Previous studies have suggested that inserting a needle may cause the locator to shift, and it is therefore recommended to insert the cannula tip at least 1 cm below the pleural surface (13,14,17,18). Prior research has shown that the coil locator is more prone to displacement and detachment in cases where the distance between the coil and pleura is less than 20 mm (12,13). However, the locator used in our study had 4 fishhook-shaped claws at the tip, which provided a firmer grip on the adjacent lung parenchyma and prevented dislocation. Additionally, the tail of the positioning line was a soft wire that minimized friction with the adjacent tissues during respiratory movement, and the tension was relatively low. Therefore, we believed that placing the locator at a greater depth was unnecessary to prevent it from detaching. To ensure that the anchor does not shift due to the patient’s own movement while waiting for surgery, we recommend performing repeated CT scans before release to confirm that the locator has successfully penetrated the pleura and entered the lungs.
In the present study, we found that needle-tract bleeding occurred more frequently when the nodule size was >0.84 cm, the nodule depth was >1.33 cm, the thickness of the chest wall at the puncture point was >3.91 cm, or the needle insertion depth was >1.01 cm. We therefore recommend that the operator should pay special attention to avoid pulmonary arterioles in the puncture path as much as possible in such cases.
The incidence of pneumothorax after surgery was relatively high (29.9%) in our study, which may be related to the CT slice thickness of 1 mm we used. A small amount of pneumothorax does not require special treatment. In our study, 1 patient developed pneumothorax and the positioner detached. This may be because the needle did not enter the lung parenchyma but did enter the thoracic cavity. Since lung tissue has a certain elasticity, the inner pleura may be compressed during puncture, but not be penetrated (13). In such cases, the surgeon should continue to insert the puncture needle to ensure that the needle tip enters the lung parenchyma.
In the present study, demographics, pulmonary nodules, and technical factors were found to have no significant impact on pneumothorax. In support of this, some studies have indicated that frequency is the only significant independent risk factor for pneumothorax (19-21). However, this study only considered patients with a single nodule, and excluded the possible impact of multiple injections. The positioning of 2 or more nodules may cause errors in measurement, which requires further study. The present cohort included patients across a wide age range (53.5±12.5 years, range 21–81 years), and our analysis indicated that age does not significantly affect the accuracy of localization or the occurrence of complications. Complications experienced by all patients were relatively mild, and did not require specialized treatment. In cases where the patient is older, has comorbid emphysema, or has low pain tolerance, postoperative care involves rest in the supine position, administration of oxygen inhalation, or pain relief medication. If needed, surgery may be scheduled in advance to minimize patient waiting time.
In the present study, surgical records were used to confirm location markers. However, considering that the success of the operation may be affected by factors related to the surgeon and individual patient, it was impossible to determine a direct relationship between the success of the operation and CT-guided localization. Despite these limitations, we believe that the depth of pulmonary nodules and the depth of the localizer are factors that affect accurate localization and complications of hemorrhage after localization. Further, we also propose that it is not necessary to insert the locator too deeply to improve the success rate of targeting pulmonary nodules for subsequent VATS.
The flexible wire hook positioner offers several advantages over traditional positioning methods: First, it surpasses dye localization by providing highly accurate nodule positioning information. Surgeons can swiftly and precisely pinpoint lung nodules with the visual tail wire and anchored tip visible in CT images, minimizing surgical errors and uncertainties. Second, its flexible tail wire is less invasive than a metal one, reducing unnecessary incisions and trauma, thus enhancing safety. Third, compared to coil localization, this device is simpler and faster to operate, alleviating patient pain and discomfort. Consequently, the soft wire claw lung nodule locator excels in precision, visualization, safety, and user-friendliness, contributing to more accurate and safer lung nodule surgeries, shortening operation times, and lessening patient pain.
This study has several limitations which should be acknowledged. This study focused solely on the influencing factors of a single positioning and did not take into account the influencing factors of secondary or multiple positioning. However, these factors will be explored in future research. In our study, we evaluated accuracy based on CT image results rather than relying on surgical records. We believe that all data in the CT image is well preserved, allowing for a comprehensive and direct analysis of the factors influencing successful localization. Nevertheless, it is important to note that there are numerous other factors that can affect the success of surgery, and it is not possible to fully correlate the localization-related factors with the surgical success rate.
Conclusions
Our results showed that the depth of the nodule and the depth of needle insertion are both important factors influencing the accuracy of soft wire claw hook positioning under CT guidance. Further, the size and depth of the pulmonary nodule, thickness of the puncture point, and depth of needle insertion affect bleeding after localization. Evaluation of these indicators should be considered when locating pulmonary nodules under CT guidance. These results will be helpful in improving the accuracy of puncture positioning, reducing repetitive operations and patient pain, and effectively reducing the operation time and postoperative risk.
Acknowledgments
We sincerely thank Xinxin Ai (Anhui Jianzhu University, Communication Visual Design) for supporting this study in the meticulous enhancement of puncture positioning needles.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1272/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1272/coif). All authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Nanjing Drum Tower Hospital. Written informed consent was provided by all patients prior to performing the CT-guided percutaneous localizations procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sato M. Precise sublobar lung resection for small pulmonary nodules: localization and beyond. Gen Thorac Cardiovasc Surg 2020;68:684-91. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, Tian DH, Black D, Yan TD. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, Puri V, Kozower BD. Long-Term Results for Clinical Stage IA Lung Cancer: Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Donington JS. Survival After Sublobar Resection Versus Lobectomy for Clinical Stage IA Lung Cancer: Analysis From the National Cancer Database. J Thorac Oncol 2015;10:1513-4. [Crossref] [PubMed]
- Rodrigues JCL, Pierre AF, Hanneman K, Cabanero M, Kavanagh J, Waddell TK, Chung TB, Pakkal M, Keshavjee S, Cypel M, Yasufuku K, Nguyen ET. CT-guided Microcoil Pulmonary Nodule Localization prior to Video-assisted Thoracoscopic Surgery: Diagnostic Utility and Recurrence-Free Survival. Radiology 2019;291:214-22. [Crossref] [PubMed]
- McDermott S, Frenk NE, Fintelmann FJ, Price MC, Ott HC, Muniappan A, Shepard JO, Sharma A. Preoperative CT-guided Fiducial Marker Placement for Surgical Localization of Pulmonary Nodules. Radiol Cardiothorac Imaging 2022;4:e210194. [Crossref] [PubMed]
- Tseng YH, Lee YF, Hsieh MS, Chien N, Ko WC, Chen JY, Lee JM, Huang PM, Lin MW, Chen JS, Chang YC. Preoperative computed tomography-guided dye injection to localize multiple lung nodules for video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S666-71. [Crossref] [PubMed]
- Hung CT, Chen CK, Chang YY, Hsu PK, Hung JJ, Huang CS, Wu YC, Hsu HS. Electromagnetic navigation-guided versus computed tomography-guided percutaneous localization of small lung nodules before uniportal video-assisted thoracoscopic surgery: a propensity score-matched analysis. Eur J Cardiothorac Surg 2020;58:i85-91. [Crossref] [PubMed]
- Zhang SF, Liu HR, Ma AL, Li EL. Preoperative computed tomography-guided localization for multiple pulmonary nodules: comparison of methylene blue and coil. J Cardiothorac Surg 2022;17:186. [Crossref] [PubMed]
- Thistlethwaite PA, Gower JR, Hernandez M, Zhang Y, Picel AC, Roberts AC. Needle localization of small pulmonary nodules: Lessons learned. J Thorac Cardiovasc Surg 2018;155:2140-7. [Crossref] [PubMed]
- Wang ZX, Li L, Zhang Z, Wang GH, Kong DM, Wang XD, Wang F. High-resolution computed tomography features and CT-guided microcoil localization of subcentimeter pulmonary ground-glass opacities: radiological processing prior to video-assisted thoracoscopic surgery. J Thorac Dis 2018;10:2676-84. [Crossref] [PubMed]
- Li CD, Huang ZG, Sun HL, Wang LT, Wang YL. CT-guided preoperative localization of ground glass nodule: comparison between the application of embolization microcoil and the locating needle designed for pulmonary nodules. Br J Radiol 2021;94:20210193. [Crossref] [PubMed]
- Xu Y, Ma L, Sun H, Huang Z, Zhang Z, Xiao F, Ma Q, Li C, Zhang X, Xie S. CT-guided microcoil localization for pulmonary nodules before VATS: a retrospective evaluation of risk factors for pleural marking failure. Eur Radiol 2020;30:5674-83. [Crossref] [PubMed]
- Seo JM, Lee HY, Kim HK, Choi YS, Kim J, Shim YM, Lee KS. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [Crossref] [PubMed]
- van Tilburg PM, Stam H, Hoogsteden HC, van Klaveren RJ. Pre-operative pulmonary evaluation of lung cancer patients: a review of the literature. Eur Respir J 2009;33:1206-15. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, Hoffstetter W, Mehran R, Roth J, Walsh G, Vaporciyan A, Swisher S. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, Hyodo T, Kouno Y, Yasui K, Mimura H, Akaki S, Kuroda M, Shimizu N, Hiraki Y. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Jin X, Wang T, Chen L, Xing P, Wu X, Shao C, Huang B, Zang W. Single-Stage Pulmonary Resection via a Combination of Single Hookwire Localization and Video-Assisted Thoracoscopic Surgery for Synchronous Multiple Pulmonary Nodules. Technol Cancer Res Treat 2021;20:15330338211042511. [Crossref] [PubMed]
- Tian Y, An J, Zou Z, Dong Y, Wu J, Chen Z, Niu H. Computed Tomography-Guided Microcoil Localization of Pulmonary Nodules: Effects of Multiple Punctures. Thorac Cardiovasc Surg 2023;71:566-72. [Crossref] [PubMed]
- Ai M. Safety and effectiveness of simultaneous localization of multiple lung nodules using coils and risk factors for pneumothorax: a retrospective study. Acta Radiol 2023;64:581-7. [Crossref] [PubMed]