
Principles and features of ultrasound hypoechogenicity in diffuse thyroid pathology
Introduction
Ultrasound (US) can effectively detect changes in the thyroid gland. In addition to detecting nodes, US can identify diffuse processes in the thyroid parenchyma, such as hypoechogenicity. This sign is present in various thyroid diseases, so researchers have made numerous attempts to determine the dependence of hypoechogenicity on the (I) nosological variant of the pathology, (II) levels of antibodies to thyroid peroxidase (TPOAb) or thyroglobulin (TGAb) and (III) hormonal metabolism (1-5). However, the results and conclusions of these studies demonstrated the non-specificity of thyroid hypoechogenicity for any particular nosology [Hashimoto’s thyroiditis, Grave’s disease (GD) and subacute de Quervain’s thyroiditis] (5-8) and the presence of gland hypoechogenicity in all hormonal metabolism variants, including euthyroidism, hypothyroidism and hyperthyroidism (4,5,9-12).
Despite the prevalence of a direct correlation between the values of laboratory parameters [levels of thyroid-stimulating hormone (TSH), TPOAb and TGAb] and thyroid hypoechogenicity intensity (4,5,9,10,12-14), some studies have reported that these laboratory indicators are not dependent on thyroid echogenicity (1,4,14-16). For example, in a study by Trimboli et al., 18.9% (46/244) patients with normal (normoechoic and homogeneous) thyroids exhibited excessive levels of TSH, TPOAb and TGAb, whereas among patients with pathological (hypoechoic and inhomogeneous) thyroids, 21.6% (51/190) exhibited normal TSH levels and 23.7% (55/190) exhibited normal TPOAb and TGAb levels (11).
As evidenced by the existing literature, contemporary studies have paid little attention to the morphofunctional backgrounds of thyroid hypoechogenicity, probably assuming that the importance of hypoechogenicity has already been understood. Therefore, some authors do not report anything regarding it (5,12), while others include only a few sentences in their articles to highlight the possible histological conditions underlying the hypoechogenicity of the thyroid parenchyma (4,12,14).
According to previous studies, understanding the nature of hypoechogenicity in the thyroid gland is usually limited to two conditions: (I) cell density (due to lymphocyte infiltration and/or reduced amount of colloid) and (II) increased saturation of vessels with blood (4,9,14). Previously (before the advent of Doppler blood flow diagnostics), hypoechogenicity of the thyroid gland was only attributed to lymphocyte infiltration (17) and follicular degeneration (18).
Some specialists suggest using diagnostic criteria and grades to evaluate hypoechogenicity (12,14). This approach has proven to be adequate for characterising changes in the processes that occur over time in the gland as pathology severity increases or decreases (14,19). However, some authors perceive and define hypoechogenicity in conjunction with heterogeneity irrespective of the morphological basis of these concepts (14,18); i.e., they refer to two different phenomena as per the common criteria of “hypoechogenicity”: (I) different degrees of homogeneous decrease of thyroid tissue echogenicity in gland lobes and (II) small areas of considerable hypoechogenicity, which they refer to as “hypoechoic foci or patches” (12).
The presented controversial conditions as well as the partial correlation of hormonal and immunological manifestations with thyroid hypoechogenicity indicate the existence of additional circumstances associated with the importance of the processes occurring in the thyroid gland, irrespective of nosological conceptions. In other words, the overall picture of thyroid processes comprising US scans and laboratory puzzles only partially reveals the reality. It lacks the essential elements and circumstances of their interaction, which are critical to understanding the fundamental importance of thyroid gland hypoechogenicity. Therefore, this study aimed to discover these elements and circumstances as well as their regular order.
Sonographic variants of thyroid hypoechogenicity
Thyroid hypoechogenicity is based on the reduced reflection of US waves. Such a phenomenon is characteristic of liquids. The more liquid is in a tissue, the more hypoechogenic is the US image of the area. The highest ultrasonic hypoechogenicity is detected in blood vessels and cysts. It is denoted by the special term “anechogenic” (lacking reflection) and appears black on US images.
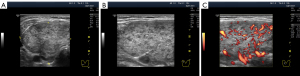
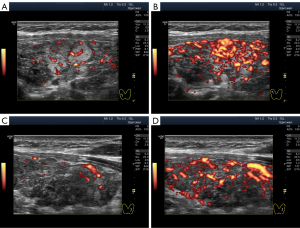
Unlike anechogenicity, hypoechogenicity appears in different degrees of dark grey, close to black, to various extents. Therefore, sonologists can visually differentiate between lower and higher hypoechogenicity at the same basic settings of the device. Furthermore, they can assess the echogenicity of the thyroid gland not only from memory and personal perception but also by comparing it with that of the parotid gland, if necessary (20). During US examination, human vision can distinguish isoechogenicity (Figure 1A) and the three variants of hypoechogenicity as follows: slight, moderate (average) and significant (Figure 1B-1D). Probably owing to this, some researchers have proposed classifying thyroid hypoechogenicity based on these (level-based) differences (4,12,14).
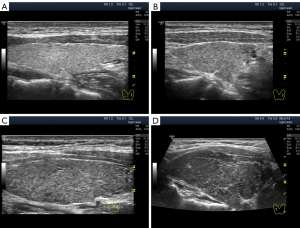
For the comparative visual methodology, we chose the basic US mode setting and the evaluation of echogenicity in percent gray. The US machine used was Logiq P9 GE Healthcare. In gray scale mode, the overall Gain was set to 60±2 dB (automatic correction was used to improve contrast). Gain in depth was selected in the middle part of the range with a slight gradual increase in depth. On a gradient black-and-white scale, different zones of echogenicity were visually selected for subsequent evaluation of US images. As a result, hyperechogenicity was defined as less than 10% gray, isoechoic as 15–20% gray, and hypoechogenicity as 25–85% gray. The hypoechogenicity zone was conventionally divided into three parts: low hypoechogenicity (25–35% gray), moderate hypoechogenicity (40–60% gray) and significant hypoechogenicity (65–85% gray). Anechoicity is defined as 90–100% gray.
The blood flow intensity was determined in power Doppler mode at a PRF of 8.1 kHz with a Gain of 22.5 dB. A longitudinal projection of each thyroid lobe at the maximum of the pulse wave was used. The projection with the largest number of vessels was selected. We used five options for blood flow intensity. Reduced blood flow was detected with 1–3 small vessels inside the lobe. Normal blood flow corresponded to 4–10 vessels in the lobe without dilatation. A slight increase in blood flow was determined with 11–20 vessels in the field of view, including some dilated ones. A moderate increase in blood flow intensity was characterized by 21–40 vessels with noticeable dilatation of some of them. A significant increase in blood flow in the lobe corresponded to more than 40 vessels in the field of view and their dilation.
Except in terms of intensity, two types of hypoechogenicity can be detected in diffuse processes in the thyroid gland via US: (I) widespread (Figure 1B-1D) and (II) segmental (Figure 2).
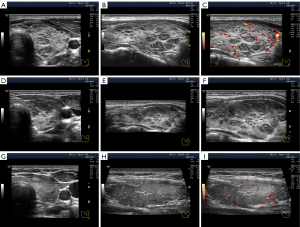
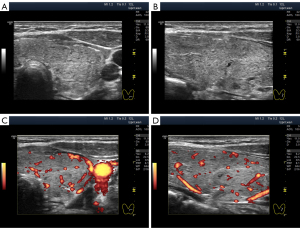
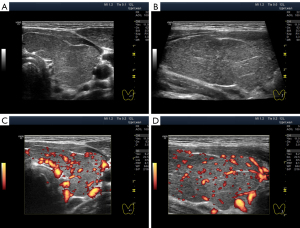
The widespread hypoechogenicity is observed as a general darkening of the tissue that may be more or less homogeneous throughout the whole lobe (right and/or left) of the thyroid gland (5). According to previous studies, this hypoechogenicity is most commonly detected in primary hypothyroidism and GD (3,12,14). In this variant of hypoechogenicity, enhanced blood flow in the thyroid gland can be simultaneously observed using the Doppler mode (Figure 3A,3B). Therefore, the widespread and especially significant hypoechogenicity (Figure 3C,3D) has sometimes been associated with the saturation of the thyroid vascular network with blood. Nevertheless, increased blood flow intensity in the thyroid parenchyma can be accompanied by isoechogenicity (Figure 4) or slight hypoechogenicity (Figures 5,6). Moreover, normal blood flow can be detected with significant hypoechogenicity (Figure 7).
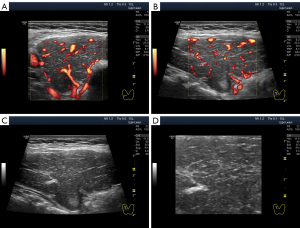
In diffuse hyperthyroidism (GD), theoretically, reduction and changes in the amount of colloid in follicles should increase cell mass concentration in thyroid parenchyma and contribute to its hypoechogenicity in US images (21). However, it is unclear whether such a correlation exists in reality only for hyperthyroidism because almost the same US pattern of echogenicity (Figures 1D,7) and/or Doppler data has been determined in primary hypothyroidism, such as in GD (22). In addition, diffuse hyperthyroidism can be accompanied by a slight hypoechogenicity of the thyroid parenchyma (Figure 5C,5D). Moreover, hypoechogenicity has been detected in euthyroidism (4-11) with normal TPOAb and TGAb levels (Figure 6C,6D). Therefore, a secondary (complementary) role of the intrafollicular process (colloid-to-cell ratio of follicles) and a leading role of the interfollicular process due to stromal swelling (fluid accumulation between follicles) are possible in the formation of widespread hypoechogenicity.
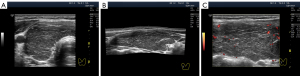
Some authors describe segmental hypoechogenicity as “patch”, “focus” or “heterogeneity” (12,14) because it involves the natural segments of the thyroid gland. The hypoechogenicity of small segments (lobules) is primarily based on tissue destruction and/or lymphocytic replacement, with subsequent possible (not obligatory) lymphocytic proliferation generating “lymphoid lobules”, which are more commonly known as “pseudonodules” or “micronodules” (23). Segmental hypoechogenicity involves isolated lobules or groups of lobules, i.e., small- and medium-sized segments that are dispersed in a lobe (Figure 8) as a single complex in a large segment (Figure 2D-2F) or as a mixed variant (Figure 2A-2C).
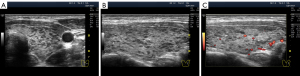
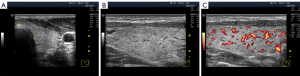
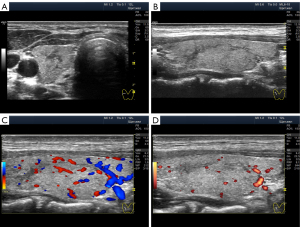
Significant hypoechogenicity in large segments may develop not only because of stromal swelling but also as a result of lymphocytic infiltration. In the latter case, the granular structure of the thyroid parenchyma is almost invisible on US images. This highly pronounced lymphocytic infiltration with thyroid tissue substitution can be observed in the Doppler mode with reduced PRF. In this Doppler setting, the blood flow in the altered area of the gland is considerably reduced compared with that of the rest of the lobe parenchyma (Figure 9).
Swelling of the thyroid stroma
Morphologists describe thyroid stromal swelling as a dilation of the venous component of microcirculation, lymphatic vessels filled with concentrated lymph and water accumulation around arteries (24-27). This fluid saturation of the thyroid parenchyma does not alter its structural organisation and may exhibit a different intensity. Therefore, it is very likely that the different degrees of hypoechogenicity and its subsequent changes (increase or decrease) within a certain period are due to changes in the degree of stromal swelling.
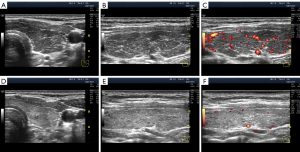
Apparently, one should distinguish between swelling along large vessels (smaller order) and microcirculation vessels (bigger order). The hypoechogenic lines of different thicknesses following the course of large vessels are observed in the former (Figure 10). In the latter, hypoechogenicity is detected in segments or throughout the parenchyma (widespread).
In addition, thyroid stromal swelling can be transient (a few hours to a day) or prolonged (several months). Short-term stromal swelling is conventionally considered to follow an acute course (28,29).
The acute stromal swelling may develop following a fine-needle aspiration biopsy or puncture of the vessels adjacent to the gland. The US image in such cases appears to be a set of thin and thick, short and extended, linear and arched, significantly hypoechogenic and/or anechogenic elements in isoechogenic tissue (28). Specialists have reported a 1.5–3-fold increase in gland volume in such cases, with different expressed degrees of painfulness on pressing and the disappearance of all pathological manifestations of the acute swelling within several hours, with the gland’s US image returning to normal the next day (particularly after applying a cold compress using non-steroidal anti-inflammatory drugs and hydrocortisone) (29). Unfortunately, these authors do not usually assess the state of blood flow in the Doppler mode, which could improve our understanding of this process. Nevertheless, the typical signs of acute thyroid swelling can be understood by perivascular swelling along the smaller-order vessels, e.g., third-order vessels.
The stromal swelling, which is thought to be associated with the microvascular network, is most commonly observed. The US signs of microvascular swelling appear as relatively homogeneous hypoechogenicity, which can last for several months (Figures 1B-1D,2G,2H,3C,3D,7,11A-11C). Concurrently, via the widespread hypoechogenicity, the usual fine mesh structure of the gland, which is the hormone-producing tissue, is evident (Figures 1-3,7,11,12). This state of the tissue is observed in primary hypothyroidism (Figures 7,11) and GD (Figure 3). Because thyroid hormone levels are often found to be adequate in the former and excessive in the latter, one might assume that the phenomenon of microvascular stromal swelling has no effect on the process of hormone production and release.
In practice, the intensity of the diffused thyroid parenchymal hypoechogenicity not only increased (from slight to moderate and significant) but also became normal (Figure 12) (14,30). In other words, the intensity of diffused hypoechogenicity is probably directly associated with the intensity of stromal swelling. In such cases, US Doppler usually reveals similar changes in parenchymal blood flow intensity and systolic peak velocity (SPV) in the thyroid arteries (TA); i.e., the blood flow intensity increases with an increase in the hypoechogenicity and vice versa and decreases with an improvement in the echogenicity (30). However, considering the absence of an absolute correlation between blood flow intensities and thyroid parenchymal hypoechogenicity (Figures 4,5), this dependency indicates the presence of not only a single regulation source for the blood supply network and stromal swelling process but also the presence of an additional circumstance leading to hypoechogenicity.
Morphofunctional foundation of thyroid hypoechogenicity
In addition to lymphocytic infiltration and proliferation in the area of thyroid parenchyma destruction, there are additional mechanisms that contribute to hypoechogenicity development. Reportedly, blood saturation of the vasculature, stromal swelling and colloid reduction in follicles are the conditions leading to the formation of thyroid parenchymal hypoechogenicity. These three phenomena are observed in GD, where the thyroid gland is under excessive functional exertion. Furthermore, GD is caused by external stress factors (31) that affect the thyroid gland via the autonomic nervous system (ANS), thereby directly impacting blood vessels and thyroid follicles (32-35).
The characteristic changes in follicles, increased thyroid blood flow and stromal swelling may be associated with increased thyroid stimulation by the ANS. Consequently, it can be considered that the conductive and/or humoral neurovegetative stimulation of the thyroid gland induces overall excessive exertion, which is visible during US examination as diffused hypoechogenicity.
The destruction process in small- and middle-sized segments of the gland (lobes and groups of lobes) occurs according to morphofunctional patterns. Such patterns include direct neuroconductive control and regulation of the thyroid parenchyma segments. These patterns are based on the natural segmentary organisation of the thyroid parenchyma and the interconnection of the ANS nerve centres with these segments.
It has been revealed that the nerve centres in the ANS comprise functional modules (36), each of which controls a specific area of an organ or part of the body (37). Using the superior sympathetic neuroganglion and thyroid nodules as examples Sudakov et al. found “dystrophic changes in ganglioneurons, foci of group fall-out of neurons and neuron-like neuropile proliferation in the ganglion compartment connected with exit poles of superior cardiac nerve containing a branch of superior thyroid artery” in humans (post-mortem) (37).
This somatotopic organisation implies that the ANS has a greater impact on certain segments of the thyroid parenchyma than on others. Hence, substantially hypoechoic segments in the thyroid parenchyma (pseudonodules/lymphoid lobes) can be considered indications of segregated tissue destruction followed by lymphocytic replacement due to local and more intense activity in response to excessive stimulation by the ANS.
Variations in blood SPV in the TA can be viewed as additional evidence of the key contribution of the ANS to thyroid activity and its changes leading to segmentary hypoechogenicity. SPV is unequal in the right and left as well as the superior and inferior TA (38). This difference is attributable to the different conditions of the functional modules in the ANS nerve centres that exhibit their respective impacts on vascular tone and particular segments of the thyroid parenchyma. Conversely, excessive and nearly equal gland stimulation by the ANS (including together with the uniform distribution of the neurovasculature in the thyroid parenchyma) causes changes that lead to widespread hypecogenicity.
It has been discovered that in the case of autoimmune thyroiditis, TA SPV alteration is associated with thyroid volume rather than TSH level (39). TA SPV values do not depend on systemic arterial pressure and the condition of the carotid arteries (40), indicating a separate regulation of thyroid vascular tone. Concurrently, an absolute dependency of TA SPV on the level of thyroid antibodies has not been reported, suggesting that TA SPV does not exhibit a direct association with the activity of the immune system (39,41). Therefore, the TA SPV value can be considered to show the effects of the ANS on thyroid processes and its compensatory reserve. TA SPV increases considerably in the presence of pathology and returns to normal following recovery (case in Figure 11). Such simultaneous recovery of blood flow and thyroid parenchyma echogenicity rules out the hypothesis regarding the involvement of angiogenesis (increased number of vessels) (39) and indicates the possibility of the nervous regulation of vascular tone and processes in the parenchyma.
In primary hypothyroidism, the blood flow intensity in the thyroid parenchyma and TA SPV is increased is excessively similar to that of GD (22); [for reference: in euthyroidism, the SPV superior thyroid arteries (STA) median was defined as 17 cm/s (42) and 26 cm/s (38)]. Additionally, this indicates ANS contribution because hypothyroidism is characterised by excessive thyroid gland stimulation (by TSH and the ANS), which differs from GD in the extent of stimulation (adequate in hypothyroidism and excessive in hyperthyroidism) (43). This is probably why, in these two hormonal conditions, we observe not only a similar increase in the blood flow intensity and velocity using the Doppler mode but also a diffused and/or segmental hypoechogenicity with practically equal intensity (Figures 3,7,11,12).
Hypoechogenicity and some increase in the thyroid blood flow have been observed in euthyroidism as well (Figures 4C,4D,7). However, in euthyroidism, blood flow and hypoechogenicity intensities usually do not reach the levels found in significant hyperthyroidism (GD) and some variations of primary hypothyroidism (22). Accordingly, hypoechogenicity can be considered the result of changes in the thyroid parenchyma caused by ANS participation.
Hypoechogenicity development along bigger order vessels during the acute swelling of the gland stroma is another indicator of peripheral ANS contribution. Such a probability is evidenced not only by vascular innervation but also by the ANS’s contribution to inflammation; alteration in neurocyte sensitivity; rapid neural response and the therapeutic effect of cold (compresses), non-steroidal anti-inflammatory drugs and hydrocortisone on nervous processes.
The hypoechogenicity associated with the swelling of the thyroid stroma probably occurs due to the phenomenon of electro-osmosis. This variant of paracellular fluid transport in epithelial tissues (including thyroid cells) was shown in the cornea (44). Furthermore, the importance of electro-osmosis for organ swelling caused by electrically active tissues (especially nervous tissues) has been demonstrated in myocardium edema (45). Thus, the possibility that the electrophysiological properties of nervous processes impact electro-osmosis in the parenchyma of various organs should not be excluded. Therefore, it is possible that nervous and electrical processes contribute to hypoechogenicity development in the thyroid gland.
Discussion
Undoubtedly, thyroid US hypoechogenicity indicates a diffuse process and is more commonly found in primary hypothyroidism and diffuse hypothyroidism (GD). Moreover, previous studies have confirmed a direct correlation between the extent of changes in hormonal metabolism and the degree of hypoechogenicity (9). These circumstances have been acknowledged by specialists as opportunities to effectively use US hypoechogenicity in diagnostics (10,46).
Concurrently, studies have reported an inferior diagnostic accuracy of thyroid hypoechogenicity. This controversy arises because, according to statistics, approximately every fifth patient with a hypoechoic thyroid gland exhibits normal levels of TSH, free T4 and TPOAb, and approximately every fifth patient with an isoechogenic thyroid gland exhibits alterations in these laboratory values (4,11). In addition, TSH levels can be excessive (hypothyroidism) or subnormal (hyperthyroidism) in both hypoechoic and isoechoic thyroid parenchyma (2,4,11,12).
Therefore, thyroid hypoechogenicity is not an accurate diagnostic criterion for evaluating hormonal metabolism variants (hypothyroidism, hyperthyroidism and euthyroidism) and immune processes (TPOAb and TGAb levels). This is because thyroid parenchymal hypoechogenicity indicates completely different processes, such as histological processes, which are only partially associated with hormonal and immunological processes.
These changes in the thyroid parenchyma are primarily due to compensatory transformation in thyrocytes, colloid and stroma owing to additional stimulation by the ANS, pituitary gland (TSH) and probably, bioelectricity. However, increase in the functional enhancement of the parenchyma does not lead to considerable changes visible as US hypoechogenicity. The compensatory reserve of the parenchyma allows it to remain isoechogenic or slightly hypoechogenic for a certain period under conditions of increased hormone production. Thus, approximately 10–20% of hypothyroidism and hyperthyroidism cases show thyroid isoechogenicity (5,8,47).
A similar conclusion can be drawn about the immune system’s response to thyrocyte depletion. The production of TPOAb and TGAb can increase without considerable lymphocytic infiltration while maintaining the conditions of thyroid isoechogenicity (4,12). Furthermore, it can be assumed that not all researchers distinguish between isoechogenicity and slight hypoechogenicity, thereby affecting their findings and conclusions. This assumption is based on the attempts of specialists to compare thyroid echogenicity with the frequent slight hypoechogenicity of salivary glands (20), as well as their insufficient experience in visually evaluating a grey scale.
Undoubtfully, excessive exertion on thyroid tissue for an extended period will cause a change (including depletion) in the compensatory reserve of its parenchyma. Moreover, in hypothyroidism, thyroid gland stimulation by the ANS and TSH increases. A similar, usually more prominent stimulation by the ANS and anti-TSH receptor antibodies is observed during diffuse hyperthyroidism. This intense stimulation of the thyroid contributes to depletion and destruction processes, ultimately leading to swelling of the stroma, lymphocytic infiltration and lymphoid proliferation (the latter can occur locally in small- and medium-sized segments of the thyroid parenchyma and is common in hypothyroidism). In this case, more pronounced hypoechogenicity is detected in the US examination. In addition to this more intense depletion leading to parenchymal destruction, the compensatory response of the immune system can increase accordingly via the increase in TPOAb and TGAb production.
With the subsequent elimination of excessive exertion of the thyroid gland is eliminated, as partially indicated by the natural normalisation of TSH levels (15), the diffused hypoechogenicity of the gland parenchyma may persist for some time (predominately due to stromal swelling). Moreover, the segmentary hypoechogenicity representing the areas of destruction and lymphocytic replacement may persist for some time even after the normalisation of the impact of the pituitary gland and ANS on the thyroid gland because the destruction processes in the lobules reverse more slowly compared with stromal swelling. Probably, in such cases and in different variations of hypoechogenicity, the normal levels of TSH and TPOAb and/or TGAb as well as normal blood flow intensity can be identified using the Doppler mode.
The proposed understanding of the thyroid hypoechogenicity model and its interconnection with hormonal and immunological processes is based on both practical and theoretical knowledge. To establish the integral pathogenic picture of this model, it was reasonably necessary to introduce concepts regarding stromal swelling, the segmentary structure of thyroid, ANS contribution, tissue compensatory reserve and outline of the precise variations of the morphological basis for hypoechogenicity of parenchyma precisely. Moreover, it is impossible to ignore the significance of the bioelectrical processes contributing to the function of the ANS, thyroid cells and interstitial tissue and stromal swelling mechanisms. Thus, the stated goal has been achieved to a certain extent.
Furthermore, as usual, not all of the specified morphofunctional factors and dependencies have been entirely investigated and understood. Such a limitation is particularly associated with an overemphasis on the level of follicular and molecular processes in the thyroid, thereby restricting the investigation of general systemic processes. Another reason is likely to be due to the attempts of the specialists to evaluate pathological processes within a single nosological entity and to contrapose nosological entities among each other (e.g., primary hypothyroidism and GD). In addition, the following points can be stated: (I) a lack of attention in studying compensation at the thyroid cell and parenchyma levels (the main focus of the studies was hormonal metabolism), (II) an erroneous and unreasonable exclusion of the ANS contribution to thyroid function and (III) the recent emergence of interest in electrobiology, especially in electro-osmosis, in medicine. Hence, to improve our understanding regarding the nature of thyroid hypoechogenicity, comprehensive knowledge based on facts and regularities is required.
Conclusions
Thus, thyroid hypoechogenicity is an US indication of a histological process exhibiting various degrees of compensatory reserve depletion in the gland parenchyma. These changes are absolutely associated with the intensity and duration of thyroid stimulation. Therefore, in most (but not all) cases, thyroid hypoechogenicity demonstrates dependency between the levels of TSH and an excess of TPOAb and TGAb.
An important contributor to exertion and depletion in the thyroid gland is the ANS. This is indicated not only by known direct morphofunctional interconnections between the nervous system and thyroid follicles and vessels but also by local changes in the natural gland segments (typical of the diffuse process) that are subordinate to different groups of ANS neurocytes. Therefore, evaluating the blood flow intensity and SPV TA is necessary to define the degree of ANS impact on the thyroid.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1357/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from the patients for their anonymized information to be published in this article.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rago T, Chiovato L, Grasso L, Pinchera A, Vitti P. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dsfunction in apparently healthy subjects. J Endocrinol Invest 2001;24:763-9. [Crossref] [PubMed]
- Mazziotti G, Sorvillo F, Iorio S, Carbone A, Romeo A, Piscopo M, Capuano S, Capuano E, Amato G, Carella C. Grey-scale analysis allows a quantitative evaluation of thyroid echogenicity in the patients with Hashimoto's thyroiditis. Clin Endocrinol (Oxf) 2003;59:223-9. [Crossref] [PubMed]
- Schiemann U, Avenhaus W, Konturek JW, Gellner R, Hengst K, Gross M. Relationship of clinical features and laboratory parameters to thyroid echogenicity measured by standardized grey scale ultrasonography in patients with Hashimoto's thyroiditis. Med Sci Monit 2003;9:MT13-7.
- Tam AA, Kaya C, Üçler R, Dirikoç A, Ersoy R, Çakır B. Correlation of normal thyroid ultrasonography with thyroid tests. Quant Imaging Med Surg 2015;5:569-74. [Crossref] [PubMed]
- Pishdad P, Pishdad GR, Tavanaa S, Pishdad R, Jalli R. Thyroid Ultrasonography in Differentiation between Graves' Disease and Hashimoto's Thyroiditis. J Biomed Phys Eng 2017;7:21-6.
- Marcocci C, Vitti P, Cetani F, Catalano F, Concetti R, Pinchera A. Thyroid ultrasonography helps to identify patients with diffuse lymphocytic thyroiditis who are prone to develop hypothyroidism. J Clin Endocrinol Metab 1991;72:209-13. [Crossref] [PubMed]
- Vitti P, Rago T, Mazzeo S, Brogioni S, Lampis M, De Liperi A, Bartolozzi C, Pinchera A, Martino E. Thyroid blood flow evaluation by color-flow Doppler sonography distinguishes Graves' disease from Hashimoto's thyroiditis. J Endocrinol Invest 1995;18:857-61. [Crossref] [PubMed]
- Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid 2000;10:251-9. [Crossref] [PubMed]
- Schiemann U, Gellner R, Riemann B, Schierbaum G, Menzel J, Domschke W, Hengst K. Standardized grey scale ultrasonography in Graves' disease: correlation to autoimmune activity. Eur J Endocrinol 1999;141:332-6. [Crossref] [PubMed]
- Loy M, Cianchetti ME, Cardia F, Melis A, Boi F, Mariotti S. Correlation of computerized gray-scale sonographic findings with thyroid function and thyroid autoimmune activity in patients with Hashimoto's thyroiditis. J Clin Ultrasound 2004;32:136-40. [Crossref] [PubMed]
- Trimboli P, Rossi F, Thorel F, Condorelli E, Laurenti O, Ventura C, Nigri G, Romanelli F, Guarino M, Valabrega S. One in five subjects with normal thyroid ultrasonography has altered thyroid tests. Endocr J 2012;59:137-43. [Crossref] [PubMed]
- Jeong SH, Hong HS, Lee JY. The association between thyroid echogenicity and thyroid function in pediatric and adolescent Hashimoto's thyroiditis. Medicine (Baltimore) 2019;98:e15055. [Crossref] [PubMed]
- Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Pedersen IB, Rasmussen LB, Ovesen L, Jørgensen T. The association between hypoechogenicity or irregular echo pattern at thyroid ultrasonography and thyroid function in the general population. Eur J Endocrinol 2006;155:547-52. [Crossref] [PubMed]
- Park JE, Hwang SM, Hwang JY, Moon JH, Yang I, Woo JY, Lee HJ. The relationship between ultrasound findings and thyroid function in children and adolescent autoimmune diffuse thyroid diseases. Sci Rep 2021;11:19709. [Crossref] [PubMed]
- Rosário PW, Bessa B, Valadão MM, Purisch S. Natural history of mild subclinical hypothyroidism: prognostic value of ultrasound. Thyroid 2009;19:9-12. [Crossref] [PubMed]
- Jung KY, Kim H, Choi HS, An JH, Cho SW, Kim HJ, Park YJ. Clinical factors predicting the successful discontinuation of hormone replacement therapy in patients diagnosed with primary hypothyroidism. PLoS One 2020;15:e0233596. [Crossref] [PubMed]
- Yoshida A, Adachi T, Noguchi T, Urabe K, Onoyama S, Okamura Y, Shigemasa C, Abe K, Mashiba H. Echographic findings and histological feature of the thyroid: a reverse relationship between the level of echo-amplitude and lymphocytic infiltration. Endocrinol Jpn 1985;32:681-90. [Crossref] [PubMed]
- Hayashi N, Tamaki N, Konishi J, Yonekura Y, Senda M, Kasagi K, Yamamoto K, Iida Y, Misaki T, Endo K. Sonography of Hashimoto's thyroiditis. J Clin Ultrasound 1986;14:123-6. [Crossref] [PubMed]
- Nanan R, Wall JR. Remission of Hashimoto's thyroiditis in a twelve-year-old girl with thyroid changes documented by ultrasonography. Thyroid 2010;20:1187-90. [Crossref] [PubMed]
- Choi I, Na DG. Can the ultrasound echogenicity of normal parotid and submandibular glands be used as a reference standard for normal thyroid echogenicity? Ultrasonography 2022;41:678-88. [Crossref] [PubMed]
- Niedziela M, Warzywoda M, Korman E. Thyroid echogeneity as a useful tool for the differential diagnosis of hyperthyroidism in the course of Graves disease and Hashimoto thyroiditis. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw 2000;6:143-50.
- Ushakov AV. Asymptomatic course of overt primary hypothyroidism with a very high peak systolic velocity of the superior thyroid arteries: a case report. Indian J Case Rep 2022;8:331-3.
- Yeh HC, Futterweit W, Gilbert P. Micronodulation: ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med 1996;15:813-9. [Crossref] [PubMed]
- Prokopchuk VS. Patomorfoz éndemicheskogo zoba Arkh Patol 1985;47:3-10. [Pathomorphism of endemic goiter].
- Kurbonov S, Davlatov IA. Pathomorphological characteristics of thyroid gland vascular system at the diffuse toxic goiter. Health care of Tajikistan 2018:29-33.
- Kurbonov S, Gulov MK, Davlatov IA. Complex changes in the structure of the thyroid gland in diffuse toxic goitter. Intern J of Applied and Fund Res 2018:46-9.
- Shadlinski VB, Ganieva GM, Jandieri KN, et al. The pecularities of thyroid vascularstromal complex changes under euthyroid and toxic forms of goiter. Med Vestn Bashk 2014;9:91-4.
- Shi L, Yan C, Xu W, Huang P. Acute diffuse and transient thyroid swelling after intravenous thrombolysis for acute ischemic stroke: A case report. Medicine (Baltimore) 2018;97:e12149. [Crossref] [PubMed]
- Zhu T, Yang Y, Ju H, Huang Y. Acute thyroid swelling after fine needle aspiration-a case report of a rare complication and a systematic review. BMC Surg 2021;21:175. [Crossref] [PubMed]
- Ushakov A. Case of Graves' disease recovery. J Clin Transl Endocrinol Case Rep 2023;100139.
- Vita R, Lapa D, Trimarchi F, Benvenga S. Stress triggers the onset and the recurrences of hyperthyroidism in patients with Graves' disease. Endocrine 2015;48:254-63. [Crossref] [PubMed]
- Cardinali DP, Stern JE. Peripheral neuroendocrinology of the cervical autonomic nervous system. Braz J Med Biol Res 1994;27:573-99.
- Young JB, Bürgi-Saville ME, Bürgi U, Landsberg L. Sympathetic nervous system activity in rat thyroid: potential role in goitrogenesis. Am J Physiol Endocrinol Metab 2005;288:E861-7. [Crossref] [PubMed]
- Silva JE, Bianco SD. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 2008;18:157-65.
- Dey M, Michalkiewicz M, Huffman LJ, Hedge GA. Thyroidal vascular responsiveness to parasympathetic stimulation is increased in hyperthyroidism. Am J Physiol 1993;264:E398-402. [Crossref] [PubMed]
- Szentágothai J. The modular architectonic principle of neural centers. Rev Physiol Biochem Pharmacol 1983;98:11-61. [Crossref] [PubMed]
- Sudakov YN, Bersenev VA, Torskaya VV. Metameric-receptorreflexotherapy. Kyiv. Health. 1986;216.
- Macedo TA, Chammas MC, Jorge PT, Pereira de Souza L, Farage L, Pegoraro BL, Pessa SU, Cerri GG. Reference values for Doppler ultrasound parameters of the thyroid in a healthy iodine-non-deficient population. Br J Radiol 2007;80:625-30. [Crossref] [PubMed]
- Bianchini Höfling D, Marui S, Buchpiguel CA, Cerri GG, Chammas MC. The End-Diastolic Velocity of Thyroid Arteries Is Strongly Correlated with the Peak Systolic Velocity and Gland Volume in Patients with Autoimmune Thyroiditis. J Thyroid Res 2017;2017:1924974. [Crossref] [PubMed]
- Chekalina NI, Burmak YH, Petrov YY, Borysova ZO, Trybrat TA, Shut SV, Kazakov YM. Justification of Increasing the Blood Flow Velocity in the Arteries of the Thyroid Gland in Autoimmune Thyroiditis as a Reflection of Endothelial Changes Due to Inflammatory Status. Curr Med Imaging Rev 2019;15:61-5. [Crossref] [PubMed]
- Sarangi PK, Parida S, Mangaraj S, Mohanty BK, Mohanty J, Swain BM. Diagnostic Utility of Mean Peak Systolic Velocity of Superior Thyroid Artery in Differentiating Graves' Disease from Thyroiditis. Indian J Radiol Imaging 2021;31:311-7. [Crossref] [PubMed]
- Kim TK, Lee EJ. The value of the mean peak systolic velocity of the superior thyroidal artery in the differential diagnosis of thyrotoxicosis. Ultrasonography 2015;34:292-6. [Crossref] [PubMed]
- Ushakov AV. Case of Conversion from Hypothyroidism to Hyperthyroidism and Back After Anti-SARS-CoV-2 vaccination. J Endocrinol Metab 2022;12:202-8.
- Fischbarg J, Hernandez JA, Rubashkin AA, Iserovich P, Cacace VI, Kusnier CF. Epithelial Fluid Transport is Due to Electro-osmosis (80%), Plus Osmosis (20%). J Membr Biol 2017;250:327-33. [Crossref] [PubMed]
- Rame JE, Müller J. Myocardial Edema Revisited in a New Paradigm of Cardiac Electrical Microcurrent Application in Heart Failure. Bioelectricity 2021;3:171-5. [Crossref] [PubMed]
- Raber W, Gessl A, Nowotny P, Vierhapper H. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid 2002;12:725-31. [Crossref] [PubMed]
- Lai SM, Chang TC, Chang CC, Kuo SH, Chen FW. Sonographic presentation in autoimmune thyroiditis. J Formos Med Assoc 1990;89:1057-62.


