
Diagnostic advantages of transrectal ultrasound with gastrointestinal agent instillation for rectal adenoma and early-stage rectal cancer: comparison of conventional transrectal ultrasound techniques
Introduction
Rectal cancer is a common malignancy of the digestive tract system that is threatening to human health despite being a long-standing, major focus of medical research (1-3). In clinical practice, the identification of a benign or malignant rectal mass is essential for the determination of the corresponding therapy, and such identification and local T staging of rectal cancer have typically been conducted by clinicians via imaging diagnosis. With advances in imaging technology, ultrasonography can be used to accurately diagnose diseases. Transrectal ultrasonography (TRUS) is widely used to diagnose rectal lesions (4-7) and is applied in the T staging of rectal cancer for accurate identification (8). Both TRUS and magnetic resonance imaging (MRI) are the common and preferred imaging examination methods in the diagnosis of rectal cancer (9-11). However, MRI has lower specificity in distinguishing between rectal adenoma and early rectal cancer as compared to TRUS. Studies on this subject have mostly focused on the local infiltration depth of middle- and late-stage rectal cancer and changes in T staging before and after neoadjuvant chemoradiotherapy (12-14); however, the diagnosis of early-stage rectal cancer and rectal adenoma via intrarectal gastrointestinal agent instillation has not been extensively examined. It has been found that the most effective method for differentiating between benign and malignant rectal polyps is to combine TRUS with the acoustic window system (AWS). The AWS extends the procedure scope and prevents polyp compression, enhancing the capability of TRUS to differentiate between benign and malignant rectal polyps (15-17). After gastrointestinal agent infusion, the intestinal cavity can be filled to prevent compression of the lesion, which is beneficial for better differential diagnosis. Due to the difficulty in displaying rectal lesions through abdominal exploration, we combine gastrointestinal drug infusion with TRUS. In this study, intrarectal gastrointestinal agent instillation was used with the conventional TRUS in clinical practice to diagnose early-stage rectal cancer and rectal adenoma, representing an innovation in this field. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1507/rc).
Methods
Study participants
A total of 68 patients admitted to the First Hospital of China Medical University from January 2015 to January 2021 and who preoperatively underwent TRUS were enrolled in this prospective study. We randomly selected patients diagnosed with rectal adenoma or early-stage rectal cancer via ultrasound. The age of the patients ranged from 25 to 82 (median 59 years) years. The cohort included 49 males and 19 females whose rectal lesions were removed in our hospital and whose pathological findings were recorded. None of the patients had any history of anal or rectal surgery, neoadjuvant chemoradiation therapy, or severe intestinal stenosis. Patients with lesions >13 cm distant from the anal margin were excluded. Before the examination, oral and written informed consents were obtained from all patients or their families. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the First Affiliated Hospital of China Medical University Medical Research Ethics Committee (No. AF-SOP-07-1.1-01).
Instruments and examination protocol
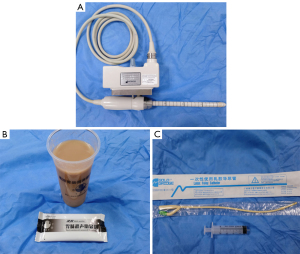
All ultrasound examinations were performed using a Hitachi Preirus Ultrasound Unit (Hitachi Medical Corporation, Tokyo, Japan) with a 360° circular-array transducer (model: EUP-R54AW-19; frequency: 5–10 MHz). There were a total of three operators in this study, all of whom have been engaged in ultrasound work for more than 10 years and have mastered TRUS technology. Before the examination, each operator was fully informed of the patients’ clinical symptoms and signs but was not aware of other examination results or pathological results. The patients were asked to fast for 6–8 h before examination, stay in the left decubitus position, and bend their hips and knees during the examination. First, the patients underwent conventional TRUS, in which the operator applied an appropriate amount of coupling agent to the surface of the probe, placed a disposable latex cover over it, applied coupling agent to the patient’s anus, and extended the probe slowly into the intestinal cavity to observe the lesion. Subsequently, the patients underwent an examination with instant gastrointestinal agent (Huzhou East Asia Medical Products Co., Ltd., Huzhou, China) instillation. For the instillation, the operator injected 60–80 mL of the prepared agent into the rectal cavity via a ureteral catheter and syringe, the catheter was pulled out, and then the probe was inserted to observe the lesion in the intestinal cavity. During the examination, the patients were advised to take a deep breath when necessary to relieve their tension and relax the sphincter ani. After the examination, the patients discharged the agent in urine.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was applied to conduct statistical analysis. The chi-squared test was used to test the detection rate of the conventional TRUS and TRUS with gastrointestinal agent instillation and to determine the consistency between the findings of these methods and the pathological findings. Additionally, the accuracy, sensitivity, and specificity of the two transrectal examination approaches were compared. We calculated the corresponding 95% confidence intervals using MedCalc 19.5.6 software. A two-sided P value <0.001 indicated statistical significance.
Results
Pathological findings and baseline information of patients
There were 130 potentially eligible patients in this study, 29 of whom were excluded. Figure S1 summarizes the reasons for excluding these 29 patients. Ultimately, 101 patients were enrolled, including 33 patients with pathological types other than rectal adenoma and early-stage rectal cancer, 27 patients with rectal adenoma, and 41 patients with early rectal cancer. Table 1 summarizes the baseline information of the lesions.
Table 1
| Patient information | Pathology, number | ||
|---|---|---|---|
| Pathological types other than rectal adenoma and early-stage rectal cancer (n=33) | Rectal adenoma (n=27) | Early-stage rectal cancer (n=41) | |
| Sex | |||
| Male | 20 | 22 | 27 |
| Female | 13 | 5 | 14 |
| Age (years) | |||
| <50 | 12 | 5 | 9 |
| ≥50 | 21 | 22 | 32 |
| Location of lesions (cm) | |||
| Low (≤5) | 18 | 10 | 11 |
| Middle (6–10) | 10 | 15 | 26 |
| High (11–12) | 5 | 2 | 4 |
| Number of lesions | |||
| Single | 28 | 10 | 41 |
| Multiple | 5 | 17 | 0 |
| Maximum diameter (cm) | |||
| <1 | 27 | 20 | 6 |
| ≥1 | 6 | 7 | 35 |
TRUS findings of the normal rectum
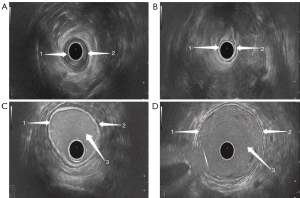
The ultrasonic probe used in TRUS and the instrument used for gastrointestinal agent injection are shown in Figure 1. TRUS provided a clear observation of the five-layer structure of the intestinal wall. These five layers, from the innermost to the outermost, respectively, were the interface layer (hyperechoic), mucosal muscle layer (hypoechoic), submucosal layer (hyperechoic), muscularis propria (hypoechoic), and serosal layer (hyperechoic). During ultrasonic probing, the interface layer is often an interface echo between the mucosa and probe or rectum contents. During the conventional TRUS, the probe was closely adhered to the mucosa (Figure 2A,2B), while during TRUS with gastrointestinal agent instillation, the interface layer was clearly shown (Figure 2C,2D).
Ultrasonography findings of rectal adenoma and early-stage rectal cancer
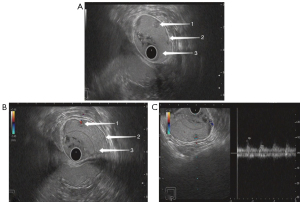
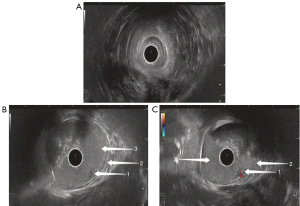
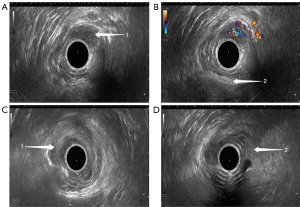
On ultrasonography, both rectal adenoma and early-stage rectal cancer appeared with a low echo, clear boundary, bulging into the intestinal cavity, and a blood flow of varying abundance. Rectal adenoma was present only in the mucosal layer, while early-stage rectal cancer was found infiltrating into the mucosal muscle layer and submucosal layer. Although it was difficult for the conventional TRUS to detect small rectal adenomas, TRUS with gastrointestinal agent instillation was able to show these lesions (Figures 3,4). For early-stage rectal cancer, both the TRUS examinations revealed the degree of the lesion; however, for most lesions, TRUS also detected the infiltration depth (Figure 5), while for a few early-stage rectal cancers, even TRUS with gastrointestinal agent instillation could not detect the infiltration depth (Figure 6).

Diagnostic efficacy of the two TRUS examinations
Of the 101 patients examined, 68 were diagnosed with rectal adenoma or early-stage rectal cancer via ultrasound. For 68 rectal lesions, the detection rate of conventional TRUS was 75.0% while that of TRUS with gastrointestinal agent instillation was 97.1%, representing a statistically significant difference (P<0.001). Conventional TRUS failed to detect 17 rectal lesions, which were adenomas. Of these, 15 were detected by TRUS with gastrointestinal agent instillation, and 2 were not detected at all. Regarding the consistency between ultrasonography and pathological findings, a difference was detected between TRUS and TRUS with gastrointestinal agent instillation. The accuracy [90.54%; 95% confidence interval (CI): 81.48–96.11%; P=1.05E−08], specificity (87.88%; 95% CI: 71.80–96.60%; P=1.09E−05), and sensitivity (92.68%; 95% CI: 80.08–98.47%; P=1.05E−08) of TRUS in diagnosing early-stage rectal cancer were consistent with the pathological findings (P<0.001) (Table 2). Table 3 shows the cross-tabulation of the TRUS and early-stage rectal cancer pathological results. The accuracy (95.95; 95% CI: 88.61–99.16%; P=3.82E−11), specificity (93.94%; 95% CI: 79.77–99.26%; P=1.31E−07), and sensitivity (97.56; 95% CI: 87.15–99.94%; P=3.82E−11) of TRUS after gastrointestinal agent infusion in diagnosing early-stage rectal cancer were consistent with the pathological findings (P<0.001) (Table 2). Table 4 shows the cross-tabulation of the results of TRUS after gastrointestinal agent infusion for early-stage rectal cancer and those of pathology. The specificity (87.88%; 95% CI: 71.80–96.60%; P=1.09E−05) of TRUS in diagnosing rectal adenomas was consistent with the pathological finding (P<0.001). The accuracy (65%; 95% CI: 51.60–76.87%; P=0.25) and sensitivity (37.04%; 95% CI: 19.40–57.63%; P=0.25) of TRUS in diagnosing rectal adenomas were not statistically significant compared to the pathological results (P>0.05) (Table 2). Table 5 shows the cross-tabulation of TRUS and rectal adenomas pathological results. The accuracy (93.33%; 95% CI: 83.80–98.15%; P=5.65E−06), specificity (93.94%; 95% CI: 79.77–99.26%; P=1.31E−07), and sensitivity (92.59%; 95% CI: 75.71–99.09%; P=5.65E−06) of TRUS after gastrointestinal agent infusion in diagnosing rectal adenomas were consistent with the pathological findings (P<0.001) (Table 2). Table 6 shows the cross-tabulation of TRUS after gastrointestinal agent infusion and the rectal adenomas pathological results. Overall, TRUS with gastrointestinal agent instillation was significantly better in the detection and diagnosis of rectal adenoma and early-stage rectal cancer than was conventional TRUS.
Table 2
| TRUS | Accuracy | Specificity | Sensitivity | |||||
|---|---|---|---|---|---|---|---|---|
| Value (95% CI), % | P | Value (95% CI), % | P | Value (95% CI), % | P | |||
| Early-stage rectal cancer | ||||||||
| Conventional method | 90.54 (81.48–96.11) | <0.001 | 87.88 (71.80–96.60) | <0.001 | 92.68 (80.08–98.47) | <0.001 | ||
| Gastrointestinal agent method | 95.95 (88.61–99.16) | <0.001 | 93.94 (79.77–99.26) | <0.001 | 97.56 ((87.15–99.94) | <0.001 | ||
| Rectal adenoma | ||||||||
| Conventional method | 65 (51.60–76.87) | >0.05 | 87.88 (71.80–96.60) | <0.001 | 37.04 (19.40–57.63) | >0.05 | ||
| Gastrointestinal agent method | 93.33 (83.80–98.15) | <0.001 | 93.94 (79.77–99.26) | <0.001 | 92.59 (75.71–99.09) | <0.001 | ||
TRUS, transrectal ultrasonography; CI, confidence interval.
Table 3
| Early-stage rectal cancer | Pathology | Total | |
|---|---|---|---|
| Positive | Negative | ||
| TRUS | |||
| + | 38 | 4 | 42 |
| − | 3 | 29 | 32 |
| Total | 41 | 33 | 74 |
Positive, early-stage rectal cancer; negative, pathological type other than rectal adenoma and early-stage rectal cancer. +, ultrasound diagnosis of early rectal cancer; −, ultrasound diagnosis other than rectal cancer. TRUS, transrectal ultrasonography.
Table 4
| Early-stage rectal cancer | Pathology | Total | |
|---|---|---|---|
| Positive | Negative | ||
| TRUS after gastrointestinal agent infusion | |||
| + | 40 | 2 | 42 |
| − | 1 | 31 | 32 |
| Total | 41 | 33 | 74 |
Positive, early-stage rectal cancers; negative, pathological type other than rectal adenoma and early-stage rectal cancer. +, ultrasound diagnosis of early rectal cancer; −, ultrasound diagnosis other than rectal cancer. TRUS, transrectal ultrasonography.
Table 5
| Rectal adenomas | Pathology | Total | |
|---|---|---|---|
| Positive | Negative | ||
| TRUS | |||
| + | 10 | 4 | 14 |
| − | 17 | 29 | 46 |
| Total | 27 | 33 | 60 |
Positive, rectal adenoma; negative, pathological type other than rectal adenoma and early-stage rectal cancer. +, ultrasound diagnosis of rectal adenoma; −, ultrasound diagnosis other than rectal adenoma. TRUS, transrectal ultrasonography.
Table 6
| Rectal adenomas | Pathology | Total | |
|---|---|---|---|
| Positive | Negative | ||
| TRUS after gastrointestinal agent infusion | |||
| + | 25 | 2 | 27 |
| − | 2 | 31 | 33 |
| Total | 27 | 33 | 60 |
Positive, rectal adenoma; negative, pathological type other than rectal adenoma and early-stage rectal cancer. +, ultrasound diagnosis of rectal adenoma; −, ultrasound diagnosis other than rectal adenoma. TRUS, transrectal ultrasonography.
Discussion
TRUS has been widely used in the diagnosis of anal and rectal lesions (18,19). One study outlined endorectal/endoanal ultrasound (ERUS/EAUS) and perineal ultrasound (PNUS), with their most crucial indications being rectal tumors and inflammatory diseases (20). The accurate preoperative determination of the local infiltration depth of rectal cancer can guide the determination of optimal therapies in clinical practice. TRUS and MRI are the existing effective methods that can determine the local infiltration depth of rectal cancer (21,22), and has its respective advantages. In some cases, conventional TRUS cannot meet the needs of clinical diagnosis. The probe of the conventional TRUS closely adheres to the rectal mucosa after it is extended into the rectum, and some gas and feces are detected in the intestinal cavity (whether perfused or not before examination), often causing lesions in the mucosal layer or small lesions in the intestinal wall to be missed or misdiagnosed. In emaciated or obese patients, the probe may not fully reach the lesion or might fail to detect them. In such cases, the advantages of TRUS with gastrointestinal agent instillation are even more apparent.
In previous studies, drinking water and coupling agent were injected into the rectal cavity to observe rectal lesions and diagnose T staging (23,24), which indicated that intrarectal instillation enables observation and accurate diagnosis of the lesion. Our study further investigated gastrointestinal instillation using instant gastrointestinal agents. When the rectal cavity was filled with the agent, the rectum could be filled up (as air inflation during an enteroscopy). The gastrointestinal agent disperses gas residues and feces in the rectal cavity, allowing for the observation of intrarectal lesions and the smooth extension of the probe. This method is superior to the injection of water or coupling agent into the rectum because the water may flows too quickly while the coupling agents may flow too slowly. Therefore, both water and coupling agents can cause discomfort in patients. Consequently, the use of gastrointestinal agent is advantageous as it is tolerable, does not harm the body, flows satisfactorily, provides a better contrast window for intrarectal lesions, disperses gas and feces inside the rectal cavity, and is discharged smoothly after examination. Moreover, the results of this study demonstrated that TRUS with gastrointestinal agent instillation has a higher detection rate, sensitivity, and accuracy for rectal lesions than does conventional TRUS, thereby effectively reducing the missed rate and misdiagnosis rate of rectal lesions.
Furthermore, in this study, conventional TRUS did not detect small rectal adenomas, while TRUS with gastrointestinal agent instillation had a significantly improved detection rate of rectal adenomas and enabled accurate diagnosis. However, TRUS with gastrointestinal agent instillation failed to detect two rectal adenomas because they were extremely small (diameter 0.2–0.3 cm and adenomatous polyps according to pathological findings) and because there was silted-up feces in the rectum. In most cases, the early-stage rectal cancers were large lesions that were identified in both examinations. However, early-stage rectal cancers with irremovable feces in the intestinal cavity were only diagnosed with TRUS and gastrointestinal agent instillation. The conventional TRUS misdiagnosed eight cases in this study (including 5 overstaged as T2 and three misdiagnosed as adenoma). The three cases misdiagnosed as adenomas had a shallow infiltration depth and a small cancerous range (pathological results showed tubular adenoma with localized malignancy). Of the five cases of ultrasound overstaging, four were diagnosed by TRUS with gastrointestinal agent instillation, which yielded results consistent with the pathological findings (which showed T1 stage rectal cancer). One case, diagnosed by TRUS with gastrointestinal agent instillation as T2 stage rectal cancer, was inconsistent with the pathological findings (which showed T1 stage rectal cancer). The reason for this erroneous ultrasound staging was the presence of a significant inflammatory infiltrate and the location of the lesion in the upper rectum. Therefore, TRUS with gastrointestinal agent instillation could diagnose lesions rather accurately when used with enteroscopy.
Some limitations to this study should be mentioned. First, there was a relatively small number of cases, only a few high rectal lesions were detected by the 360° circular-array probe up to 18 cm depth, and only a few patients facilitated the extension of the probe into the depth of 18 cm in practical application. Second, some studies have evaluated the diagnostic performance of endorectal ultrasound and shear-wave elastography in patients with complex rectal adenoma or early rectal cancer (25). Therefore, in future clinical research, we also plan to examine the use of shear-wave elastography and recruit more cases.
Conclusions
Conventional TRUS can accurately diagnose early rectal cancer. TRUS with gastrointestinal agent instillation further enhances the diagnostic accuracy of early rectal cancer, reducing the missed rate and misdiagnosis rate. It is also advantageous due to its convenient and broad application, low cost, and simple operation. Therefore, its extensive use in clinical practice is warranted.
Acknowledgments
The authors would like to thank Professor Pengtao Guo for his theoretical support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1507/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1507/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Before the examination, oral and written informed consents were obtained from all patients or their families. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the First Affiliated Hospital of China Medical University Medical Research Ethics Committee (No. AF-SOP-07-1.1-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Curvo-Semedo L. Rectal Cancer: Staging. Magn Reson Imaging Clin N Am 2020;28:105-15. [Crossref] [PubMed]
- Oronsky B, Reid T, Larson C, Knox SJ. Locally advanced rectal cancer: The past, present, and future. Semin Oncol 2020;47:85-92. [Crossref] [PubMed]
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233-54. [Crossref] [PubMed]
- El Hajj II, DeWitt J, Sherman S, Imperiale TF, LeBlanc JK, McHenry L, Cote GA, Johnson CS, Al-Haddad M. Prospective evaluation of the performance and interobserver variation in endoscopic ultrasound staging of rectal cancer. Eur J Gastroenterol Hepatol 2018;30:1013-8. [Crossref] [PubMed]
- Ye D, Zhu Z, Chen F, Lie C, Li W, Lin Y, Qiu S. Correlation Between Endorectal Ultrasound and Magnetic Resonance Imaging for Predicting the Circumferential Resection Margin in Patients With Mid-Low Rectal Cancer Without Preoperative Chemoradiotherapy. J Ultrasound Med 2020;39:569-77. [Crossref] [PubMed]
- Zhang G, Cui L, Wang X, Chu Y, Wu C. Analysis of the Diagnostic Value of Transrectal Ultrasonography for Rectal Submucosal Lesions. Altern Ther Health Med 2023;29:288-93.
- Zhang Q, Yan HL, Lu Q, Luo Y. Value of contrast-enhanced ultrasound in deep angiomyxoma using a biplane transrectal probe: A case report. World J Gastroenterol 2023;29:4214-21. [Crossref] [PubMed]
- Feng Y, Peng C, Zhu Y, Liu L. Biplane transrectal ultrasonography plus ultrasonic elastosonography and contrast-enhanced ultrasonography in T staging of rectal cancer. BMC Cancer 2020;20:862. [Crossref] [PubMed]
- Liu M, Yin S, Li Q, Liu Y, Pei X, Han F, Li AH, Zhou J. Evaluation of the Extent of Mesorectal Invasion and Mesorectal Fascia Involvement in Patients with T3 Rectal Cancer With 2-D and 3-D Transrectal Ultrasound: A Pilot Comparison Study With Magnetic Resonance Imaging Findings. Ultrasound Med Biol 2020;46:3008-16. [Crossref] [PubMed]
- Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019;39:367-87. [Crossref] [PubMed]
- Klotz L, Chin J, Black PC, Finelli A, Anidjar M, Bladou F, Mercado A, Levental M, Ghai S, Chang SD, Milot L, Patel C, Kassam Z, Moore C, Kasivisvanathan V, Loblaw A, Kebabdjian M, Earle CC, Pond GR, Haider MA. Comparison of Multiparametric Magnetic Resonance Imaging-Targeted Biopsy With Systematic Transrectal Ultrasonography Biopsy for Biopsy-Naive Men at Risk for Prostate Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021;7:534-42. [Crossref] [PubMed]
- Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, Hui B, Liu R, Ma H, Ren J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci 2016;12:1022-31. [Crossref] [PubMed]
- Ren Y, Ye J, Wang Y, Xiong W, Xu J, He Y, Cai S, Tan M, Yuan Y. The Optimal Application of Transrectal Ultrasound in Staging of Rectal Cancer Following Neoadjuvant Therapy: A Pragmatic Study for Accuracy Investigation. J Cancer 2018;9:784-91. [Crossref] [PubMed]
- Xiao Y, Xu D, Ju H, Yang C, Wang L, Wang J, Hazle JD, Wang D. Application value of biplane transrectal ultrasonography plus ultrasonic elastosonography and contrast-enhanced ultrasonography in preoperative T staging after neoadjuvant chemoradiotherapy for rectal cancer. Eur J Radiol 2018;104:20-5. [Crossref] [PubMed]
- Jensen DRK, Jaensch C, Madsen AH. The accuracy of trans rectal ultrasonography (TRUS) in early-stage rectal cancer or benign adenomas. Scand J Gastroenterol 2019;54:603-8. [Crossref] [PubMed]
- Gao Y, Hu JL, Zhang XX, Zhang MS, Zheng XF, Liu SS, Lu Y. Accuracy of endoscopic ultrasound in rectal cancer and its use in transanal endoscopic microsurgery. Minim Invasive Ther Allied Technol 2020;29:90-7. [Crossref] [PubMed]
- Rafaelsen SR, Kronborg O, Fenger C, Drue H. Comparison of two techniques of transrectal ultrasonography for the assessment of local extent of polypoid tumours of the rectum. Int J Colorectal Dis 1996;11:183-6. [Crossref] [PubMed]
- Deng XH, Tang LN, Shen YH, Huang WQ, Chen YJ. Value of dual contrast-enhanced ultrasound in the preoperative T staging of rectal carcinoma. Zhonghua Yi Xue Za Zhi 2017;97:684-6.
- Karanikas I, Koutserimpas C, Siaperas P, Skarpas A, Karoubalis J, Velimezis G. Transrectal ultrasonography of perianal fistulas: a single center experience from a surgeon's point of view. G Chir 2018;39:258-60.
- Nuernberg D, Saftoiu A, Barreiros AP, Burmester E, Ivan ET, Clevert DA, Dietrich CF, Gilja OH, Lorentzen T, Maconi G, Mihmanli I, Nolsoe CP, Pfeffer F, Rafaelsen SR, Sparchez Z, Vilmann P, Waage JER. EFSUMB Recommendations for Gastrointestinal Ultrasound Part 3: Endorectal, Endoanal and Perineal Ultrasound. Ultrasound Int Open 2019;5:E34-51. [Crossref] [PubMed]
- Kalisz KR, Enzerra MD, Paspulati RM. MRI Evaluation of the Response of Rectal Cancer to Neoadjuvant Chemoradiation Therapy. Radiographics 2019;39:538-56. [Crossref] [PubMed]
- Boot J, Gomez-Munoz F, Beets-Tan RGH. Imaging of rectal cancer. Radiologe 2019;59:46-50. [Crossref] [PubMed]
- Kim S, Lim HK, Lee SJ, Choi D, Lee WJ, Kim SH, Kim MJ, Lim JH. Depiction and local staging of rectal tumors: comparison of transrectal US before and after water instillation. Radiology 2004;231:117-22. [Crossref] [PubMed]
- Wu G, Gao Y, Hong H, Wang Y, Li J, Wu W, Liang D. Diagnostic Value of 360° Circular Scanning Transrectal Ultrasound Combined with Couplant Perfusion For Rectal Tumors. Chinese Journal of Ultrasound in Medicine 2020;36:1028-31.
- Loft MK, Pedersen MRV, Lindebjerg J, Rahr HB, Rafaelsen SR. Endorectal Ultrasound Shear-Wave Elastography of Complex Rectal Adenoma and Early Rectal Cancer. Diagnostics (Basel) 2022;12:2166. [Crossref] [PubMed]


