
Computed tomography angiography-confirmed aortic in-stents floating thrombus after endovascular stenting: a retrospective study
Introduction
Aortic stenting is a crucial procedure for treating aortic diseases such as aneurysms, dissections, coarctation, and ulceration. This procedure has shown promising results (1-4). However, some of the common complications after the endovascular intervention include restenosis, endoleak, thrombosis, dislocation, and fracture of stent graft (3,4). To detect and correct serious complications and prevent death, several guidelines recommend lifelong postoperative imaging follow-up, such as duplex ultrasonography and computed tomography angiography (CTA) examination (1,3,4).
Postoperative aortic in-stent floating thrombus (ASFT) is a rare complication that can increase the risk of severe distal embolism (5). ASFT can present with nonspecific symptoms and can be identified through routine imaging examinations. Patients may experience abdominal or chest pain due to underlying visceral infarction (6). The diagnosis of ASFT is primarily based on imaging findings, such as CTA examinations. On CTA imaging, it is common to find a mural thrombus with free-floating components within the aorta. Yang and colleagues introduced the break-off risk ratio (boRR) as a parameter to predict the risk of the ASFT breaking away from the vessel wall (5). The boRR is defined as the length ratio of the floating portion. Despite ongoing research, the causes and treatment of ASFT remain unclear (5,6).
This study aimed to investigate the evolution of CTA imaging features in patients with ASFT after receiving aortic endovascular implantation of stent graft. The treatment protocol was also investigated. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1446/rc).
Methods
Study population
This study was a longitudinal, retrospective study. The consecutive patients with aortic diseases who received endovascular implantation of stent graft at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from January 2015 to December 2022 were screened. The aortic diseases were confirmed through imaging findings and clinical symptoms. Patients with ASFT, confirmed by CTA examination during the follow-up period, were included in the study. Clinical features, as well as initial and follow-up CTA findings, were reviewed.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (2023-0815), and individual consent for this retrospective analysis was waived.
Surgery procedure
A thorough history and physical examination were conducted on all patients, with special focus on aortic disease and the patient’s performance status. A recent CTA scan was performed on all patients to evaluate vessel anatomy and determine the optimal stenting site. Prior to the operation, written consent from the patient or their family was obtained. During the operation, 3,000–5,000 units of heparin were administered as a standard practice, with smaller doses given to patients with injuries or elevated bleeding risks. The surgical procedure was consistent with the previously described protocol (1,2,7-9).
In this study, the endografts used were Aegis (MicroPort, Shanghai, China), Talent (Medtronic, Minneapolis, MN USA), and Valiant (Medtronic, Minneapolis, MN, USA). The size of grafts used was 5–20% larger than the maximum diameter (inside-inside) of the aorta at the proximal landing zone. Tapered stents or overlapping tapered stents were used when the diameter of the distal landing zone was too small. The choice of grafts was based on anatomical conditions, operator experience, and equipment availability. The proximal sealing length was determined to be at least 1.5 cm. Following aortic stenting, bridging stent grafts were inserted between the fenestration or branch and each target vessel as required. To prevent thrombus formation in patients at risk (such as those with clotting disorders, active cancer, or infection), an oral dual antiplatelet pharmacotherapy consisting of aspirin 75 mg and/or clopidogrel 75 mg per day was prescribed postoperatively for at least 3 months.
CTA protocol and image analysis
The CTA studies were conducted using a multislice helical computed tomography (CT) scanner (Somatom Definition AS Siemens, Erlangen, Germany) in accordance with our standard of care. We used prospective electrocardiogram (ECG) triggering for image acquisition, with 70% triggering when the heart rate was below 70 beats/min and 40% triggering when the heart rate was above 70 beats/min. Scanning parameters followed standard CTA techniques from the supra-aortic vessel level to the bilateral femoral arteries. The images were processed using Syngo.via (Siemens), which involved volume-rendering technique, maximum intensity projection, and multiplanar reconstruction.
The definition of ASFT stated that the thrombus was attached to one or more points on the blood vessel wall at its proximal end, while the distal end was free and moved with the blood flow. In contrast, a mural thrombus was defined as a thrombus that was attached to the inner wall of the stent and was not free at the distal end. The observed parameters included ASFT location, morphology, size, the involved aorta, concomitant visceral or vascular embolism, graft placement, and the dynamic changes of these signs during the follow-up period. The imaging of ASFT could be categorized into two types. Type 1 appeared striated, irregular, or sheet-like, and was usually short. Type 2 (classical type) was a free-floating middle section in the cavity with one or multiple points of attachment to the thickened inner wall of stent grafts. Two senior radiologists with 10 years of experience in radiology independently assessed the CT features using both axial CT images and multiplanar reconstructions. The images were evaluated independently in a blind fashion with a time gap of 2 weeks to minimize recall bias. In case of disagreements, readers discussed and reached consensus.
Follow-up
The preferred imaging modality for follow-up examinations was the CTA examination, except for patients with contraindications such as a known allergy to iodinated contrast material or renal dysfunction. The follow-up CTA scans were analyzed in a blinded fashion by the same experienced readers, as described for the baseline evaluation, to evaluate disease progression between the follow-up and baseline scans. All patients were scheduled for follow-up aortic CTA examinations and clinical symptom assessments at our institution. Additionally, detailed out-of-hospital histories and laboratory tests were requested if necessary.
Statistical analysis
Categorical data were presented as the number of patients and percentage, while continuous data were expressed as mean ± standard deviation and median (range) for non-normally distributed variables. The Mann-Whitney U test was utilized for variable comparison when necessary, and all statistical analyses were conducted using SPSS software (version 26.0; SPSS Inc., Chicago, Illinois, USA). A two-tailed P value less than 0.05 was considered statistically significant.
Results
Patient characteristics
In our study, 1,626 patients were screened and 72 cases were diagnosed with thrombus in the grafts. Finally, 10 cases were diagnosed with ASFT with CTA examination, resulting in an incidence rate of 0.62% (10/1,626) (Figure 1). The study included nine men and one woman with a median age of 54 years (range, 23–67 years). Among these patients, three had abdominal aortic aneurysm (AAA), three had type B aortic dissection (TBAD), and one each had type A aortic dissection (TAAD), abdominal aortic dissection (AAD), coarctation of the thoracic aorta (CoTA), and thoracic aortic ulcer. Seven patients had a history of hypertension and four patients developed atherosclerosis before admission. Tables 1,2 summarize the patients’ characteristics and the results of main laboratory tests before surgery. The fibrinogen (FIB), prothrombin time (PT), and D-dimer levels showed a higher trend compared to the standard value.
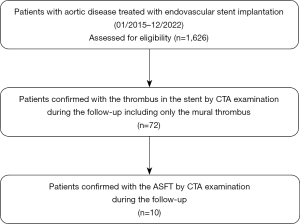
Table 1
| Case | Age (years) | Sex | Main complaint | Other symptoms | Clinical histories | Physical examination (positive signs) |
Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 39 | M | Right lower extremity numbness | – | Hypertension, hyperuricemia, gouty arthritis and high blood sugar | – | TBAD |
| 2 | 45 | M | Chest pain | Chest tightness and dizziness | Hypertension | – | TAAD |
| 3 | 67 | M | Lower back pain | – | Hypertension and atherosclerosis | – | AAA |
| 4 | 60 | M | Abdominal discomfort | – | Hypertension, diabetes, coronary heart disease and atherosclerosis | – | AAD |
| 5 | 67 | F | Frequent persistent chest back pain | Dizziness and nausea | Hypertension, coronary heart disease and autoimmune hepatitis | – | TBAD |
| 6 | 23 | M | High blood pressure | Dizziness | – | Enhancement pulsation of bilateral carotid arteries and radial arteries and the weaken pulsation of the dorsalis pedis arteries of both lower limbs | CoTA |
| 7 | 66 | M | Chest tightness | Headache and dizziness | Hypertension and atherosclerosis | – | Thoracic aortic ulcer |
| 8 | 51 | M | Chest pain | Lower back pain | Atrophic gastritis | – | TBAD |
| 9 | 57 | M | Abdominal discomfort | – | Atherosclerosis | – | AAA |
| 10 | 43 | M | Abdominal pain | – | Hypertension | – | AAA |
M, male; F, female; TBAD, type B aortic dissection; TAAD, type A aortic dissection; AAA, abdominal aortic aneurysm; AAD, abdominal aortic dissection; CoTA, coarctation of the thoracic aorta.
Table 2
| Variables | Before | Days 5–7 | P value |
|---|---|---|---|
| TT (Ref, 14.0–21.0) (s) | 16.67±1.72 | 18.08±1.80 | 0.112 |
| FIB (Ref, 2.0–4.0) (g/L) | 4.61±1.33 | 4.59±1.19 | 0.597 |
| APTT (Ref, 28.0-43.5) (s) | 40.33±3.64 | 41.76±5.07 | 0.762 |
| INR (Ref, 0.80-1.31) | 1.03±0.09 | 1.13±0.18 | 0.289 |
| PT (Ref, 11.0-16.0) (s) | 13.10±0.68 | 13.97±1.77 | 0.426 |
| D-dimer (Ref, <0.5) (mg/L FEU) | 0.97±0.659 | 5.02±1.91 | <0.001 |
| Plt (Ref, 125-350) (109/L) | 323.4±148.92 | 296.2±112.20 | 0.880 |
Data are presented as mean ± standard deviation. Ref, reference; TT, thrombin time; FIB, fibrinogen; APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; FEU, fibrinogen equivalent units; Plt, platelet.
Imaging findings
In the 10 patients, 21 floating thrombi were observed in the cavity with CTA. The ASFT length ranged from 0.15 to 2.75 cm with an average of 1.18 cm, and boRR ranged from 1.2 to 4.3. The median time interval from surgery to thrombosis was 3.5 months (range, 1–25 months). A total of 36 CTA scans were performed in the 10 cases during hospitalization and follow-up.
Atherosclerotic plaque was confirmed in four patients before surgery through CTA imaging, which showed that the location of thrombus was closely related to the location of atherosclerotic plaque. Based on the CTA examination, 18 ASFTs were basically irregular and with flaky filling defects with short and striped appearance, which were classified as type 1 (Figure 2). Three ASFTs had a pedunculated mass with multiple attachments to the inner wall of stent grafts, with the distal segment free-floating, classified as type 2 (Figure 3). As shown in Figure 4 (type 2), for the same patient, cine images of the thrombus with multiphase reconstruction from 0% to 90% of the cardiac cycle were obtained with intervals of 10% (Video S1). Table 3 displays the imaging findings of all patients.

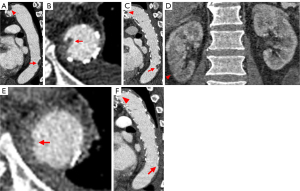
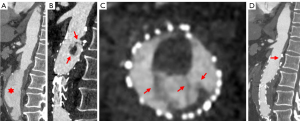
Table 3
| Case | Number of lesions | Diagnosis before the endovascular intervention | Thrombus location | Morphology/boRR | Mobilization dynamics | Concomitant embolism | Time interval between the endovascular intervention and the occurrence of the thrombus (months) | Treatment method | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | TBAD | Distal part of the stent | Striped in appearance, the head and the tail part connected to the inner wall of the stent while the middle part floating (0.32 cm) | Mainly stable state with partial components floating | – | 25 | Conservative treatment (clopidogrel) | Disappeared without new-onset embolism |
| 2 | 3 | TAAD | Original part of the stent | Two irregular, flaky filling defects (0.59 cm, 0.45 cm); multiple attachments to the stent inner wall with the distal segment free-floating in another one (1.62 cm × 0.60 cm)/2.7 | Median partial component floating along with the blood flow | Partial splenic infarction | 5 | Conservative treatment (Warfarin, adjusted according to INR level; aspirin) | Disappeared without new-onset embolism |
| 3 | 1 | AAA | Original coarctation part of the stent | Pedunculated mass (0.15 cm × 0.09 cm × 0.11 cm) with three attachment points between the free-floating distal segment and the mural thrombus/4.3 | Distal segment floating along with the blood flow | – | 2.5 | Conservative treatment (aspirin, clopidogrel) | Disappeared without new-onset embolism |
| 4 | 2 | AAD | Origin and distal part of the stent | Short and striped in appearance with a small branch distal to the lesion (0.48 cm, 1.39 cm)/1.2, 2.9 | Along with the direction of the blood flow | – | 1 | Conservative treatment (clopidogrel) | Disappeared with infarction of the inferior part of the right kidney |
| 5 | 3 | TBAD | Original part of the stent | Striped in appearance, the head and the tail part connected to the inner wall of the stent while the middle part floating (1.36 cm, 1.17 cm, 0.73 cm) | Mainly stable state with partial components floating | – | 24 | Conservative treatment (aspirin) | Remained stable without significant changes or new-onset embolism |
| 6 | 2 | CoTA | Original part of the stent | Flaky filling defect (0.5 cm); striped in appearance (2.40 cm × 0.17 cm) and the head and the tail part connected to the inner wall of the stent while the middle part floating with multiple attach points between the distal segment and the thickened inner wall of the stent | Along with the direction of the blood flow | – | 3 | Regular follow-up without antithrombotic therapy | Remained stable without significant changes or new-onset embolism |
| 7 | 3 | Thoracic aortic ulcer | Middle part of the stent | Striped in appearance, the head and the tail part connected to the inner wall of the stent while the middle part floating (2.19 cm, 0.78 cm, 0.60 cm) | Perpendicular to the direction of the blood flow | – | 4 | Regular follow-up without antithrombotic therapy | Remained stable without significant changes or new-onset embolism |
| 8 | 4 | TBAD | Original part of the stent | Two irregular, flaky filling defects (1.19 cm, 1.97 cm); two striped in appearance, the head and the tail part connected to the inner wall of the stent (1.16 cm, 2.09 cm)/1.4, 1.7 | Median partial component floating along with the blood flow | – | 2 | Conservative treatment (aspirin) | Remained stable without significant changes or new-onset embolism |
| 9 | 1 | AAA | Distal part of the stent | Irregular, short, flaky filling defects (0.94 cm)/1.3 | Along with the direction of the blood flow | – | 1 | Regular follow-up without antithrombotic therapy | Disappeared without new-onset embolism |
| 10 | 1 | AAA | Distal part of the stent | Striped in appearance, the head and the tail part connected to the inner wall of the stent while the middle part floating (2.75 cm) | Along with the direction of the blood flow | – | 18 | Receiving thrombolysis in the process of EVAR for another AAA | Disappeared without new-onset embolism |
ASFT, aortic in-stents floating thrombus; CTA, computed tomography angiography; boRR, break-off risk ratio; TBAD, type B aortic dissection; TAAD, type A aortic dissection; INR, international normalized ratio; AAA, abdominal aortic aneurysm; AAD, abdominal aortic dissection; CoTA, coarctation of the thoracic aorta; INR, international normalized ratio; EVAR, endovascular aortic aneurysm repair.
Outcomes
Ten patients underwent technically successful endovascular intervention without any cases being converted to open surgery. During a median follow-up period of 45 months (range, 22–88 months), there were no deaths during the perioperative period and none of the patients showed any late endoleak, graft migration, paraplegia, or reintervention. The details of the endovascular intervention are summarized in Table 4. There was no statistically significant difference in laboratory examinations after the endovascular intervention compared to that before the treatment, except for a statistically significant increase in D-dimer levels during the postoperative period (P<0.001) (Table 2). One patient developed partial splenic infarction after confirmation of ASFT and received conservative treatment with Warfarin and aspirin. The extent of splenic embolization did not increase further.
Table 4
| Variables | Value |
|---|---|
| Operation time (minutes) | 52.4±6.4 |
| Stent type | |
| Aegis (Microport) | 2 |
| Talent (Medtronic) | 1 |
| Valiant (Medtronic) | 7 |
| Stent length (mm) | 158±32.5 |
| Oversizing (%) | 10±5 |
Data are presented as mean ± standard deviation or number.
Regarding the aortic stent thrombosis, six patients were given conservative medical treatment. Two patients were administered clopidogrel, while two patients received aspirin. One patient received both aspirin and clopidogrel as part of their treatment, whereas the other patient was administered aspirin and warfarin. The dosage of aspirin and clopidogrel had been predetermined, and the dosage of warfarin was adjusted according to the international normalized ratio (INR) level. Among them, four patients had their lesions disappeared without any new-onset embolism during the follow-up period. One patient had a stable lesion without any significant changes or new-onset embolism, while another patient developed infarction of the inferior part of the right kidney during subsequent follow-up.
Out of the three patients who did not receive antithrombotic therapy, two showed stable thrombus without any significant changes or new-onset embolism. In one patient, the lesion disappeared without any distal organ embolism.
Only one case required another endovascular treatment for the progressively enlarged AAA, which was successfully treated during the surgery. The thrombus disappeared without any new embolism or thrombus during the follow-up after the surgery as well (Table 3).
Discussion
The incidence of ASFT is rare, with only a few cases reported in previous studies. Song et al. reported only one case of ASFT out of 34 patients (10), while Yang et al. showed a much lower incidence rate of ASFT compared to primary aortic mural thrombus (0.45%) (5). Our study also identified a low incidence rate of ASFT (0.62%), highlighting the rarity of this disease. In this study, we identified the incidence and changes of image-based features of ASFT after the procedure and outlined a treatment strategy to ensure timely and effective treatment. Our findings serve as a valuable resource for radiologists and clinicians to better understand the post-procedural changes and improve patient outcomes.
ASFT can increase the risk of severe distal embolization, particularly in cases of pedunculated thrombus (6). Yang et al. proposed boRR to assess the stability of the floating mass, we found that the thrombus with an irregular appearance was difficult to define using this method (5), which limited its application in our study. Additionally, our cohorts only identified one patient with distal embolization during the follow-up period, therefore, the optimal threshold of the boRR for defining the risk of break is still uncertain. Further validation is necessary using larger clinical data sets.
In this research, it was observed that most ASFTs (18/21) had a striped appearance, which is a novel finding. The location of the thrombus was found to be closely related to that of the atherosclerotic plaque before treatment. Therefore, our speculation suggests a potential close association between the location and presence of the ASFT and the plaque, which has not been previously reported. Another possible hypothesis that cannot be excluded is that the blood flow slows down in a certain area and clot formation occurs. The graft infolding due to excessive oversize may cause the disturbance of blood flow, which may lead to floating thrombus (11). It is not possible to investigate the oversizing ratio at the thrombus formation point and compare to that of non-thrombus area due to the limited sample size in this study. Further studies with larger cohorts are necessary to validate two hypotheses.
Accurate detection of ASFT is crucial for diagnosis, treatment, and prognosis improvement. With the advancements in CT technology, lesions inside aortic grafts could be clearly and tridimensionally demonstrated with greater spatial and temporal resolution, lower radiation, and powerful post-processing techniques. Aortic CTA examinations with electrocardiograph gating and multiplanar reconstruction of images can display graft and ASFT features such as morphology, size, location, and concomitant visceral or vascular embolism (12,13). This study accurately displayed stems and cross-linking sites by combining different sections and phases of CTA images. It is important to note that the complete features of the lesion cannot be visualized in a single-phase CT scan or a single image. This highlights the superiority of CT reconstruction technology. However, by obtaining cine images at intervals of 10% from 0% to 90% of the cardiac cycle, the lesion can be visualized. Cine-CT images, which are obtained using the retrospective ECG-gating protocol with the reconstruction of the multiphases axial CT data, can depict the motion of the thrombi over a cardiac cycle in an arbitrary plane. This helps to more fully characterize the ‘floating’ feature of the thrombi.
A variety of reasons that may lead to the formation of ASFT, including concomitant disorders such as atherosclerosis, hypercoagulable states in patients [especially those with aortic calcifications (5)], and potential vascular endothelial injury or abnormal hemodynamic properties following implantation of graft (14). This study found that four patients with confirmed atherosclerosis developed thrombus in the graft, indicating the need for early prophylactic anticoagulant therapy. Additionally, in six other patients, postoperative hemodynamic changes were likely the cause of ASFT due to increased pressure leading to stenosis. Abnormal blood flow conditions, such as turbulence and eddies, can also impact wall shear stress and result in local thrombosis at the site (5).
Currently, there are no established therapeutic protocols or guidelines for the management of ASFT. Treatment options are largely dependent on the patient’s condition and the clinician’s experience. Conservative medical treatment or invasive procedures such as interventional operations or surgery are possible therapies. However, previous studies had reported the occurrence of antiplatelet drug ‘resistance’ in patients receiving antiplatelet therapy. This meant that despite the medication, thrombotic events continued to occur and the patient did not benefit from the treatment (15). According to a study, 7% of patients on aspirin and 27% of patients on clopidogrel were found to be nontherapeutic (16). A laboratory test to evaluate platelet function can help identify these patients. For those patients, replacing the antiplatelet therapy with other drugs or increasing the dose of the original medication can be effective. In some cases, anticoagulant therapy may also be added if necessary.
The study has a few limitations. Firstly, although we highlighted the advantages of CTA examination, large-scale epidemiologic studies have not yet assessed the effects of exposure to multiple CT studies. The risks of radiation exposure and the use of iodinated contrast material should be carefully evaluated for each individual. Secondly, we did not compare different imaging modalities such as CTA, duplex ultrasonography, and magnetic resonance imaging (MRI) by evaluating their pros and cons. Finally, due to the low incidence rate of ASFT, the sample size was relatively small, which may result in an inevitable bias.
Conclusions
In conclusion, our research highlights the importance of recognizing ASFT as a rare yet significant source of peripheral embolism, which should be carefully monitored by clinicians and radiologists. Our study classified the imaging manifestation of ASFTs into two distinct types, with CTA examination proving to be a safe and preferred imaging modality for evaluating their evolution and prognosis. Conservative treatment may be an effective method for managing asymptomatic ASFT. Long-term individualized treatment and follow-up are crucial for improving patient outcomes.
Acknowledgments
The abstract of this manuscript was submitted to and accepted for the Society of Cardiovascular Computed Tomography 2023 as a poster presentation (https://doi.org/10.1016/j.jcct.2023.05.041).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1446/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1446/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (2023-0815), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schanzer A, Oderich GS. Management of Abdominal Aortic Aneurysms. N Engl J Med 2021;385:1690-8. [Crossref] [PubMed]
- Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol 2021;18:331-48. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg 2009;37:149-59. [Crossref] [PubMed]
- Yang S, Yu J, Zeng W, Yang L, Teng L, Cui Y, Shi H. Aortic floating thrombus detected by computed tomography angiography incidentally: Five cases and a literature review. J Thorac Cardiovasc Surg 2017;153:791-803.
- Piffaretti G, Tozzi M, Mariscalco G, Bacuzzi A, Lomazzi C, Rivolta N, Carrafiello G, Castelli P. Mobile thrombus of the thoracic aorta: management and treatment review. Vasc Endovascular Surg 2008;42:405-11. [Crossref] [PubMed]
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. [Crossref] [PubMed]
- Sultan S, Concannon J, Veerasingam D, Tawfick W, McHugh P, Jordan F, Hynes N. Endovascular versus conventional open surgical repair for thoracoabdominal aortic aneurysms. Cochrane Database Syst Rev 2022;4:CD012926. [Crossref] [PubMed]
- Riambau V, Böckler D, Brunkwall J, Cao P, Chiesa R, Coppi G, et al. Editor's Choice - Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2017;53:4-52. [Crossref] [PubMed]
- Song Y, Wang D, Zhang W, Fang H, Zhu H, Meng L, Bi Y, Lu X, Shi H. Evaluation of spiral CT angiography in postoperative follows up of endoluminal stent grafting with aortic disease Chinese Journal of Interventional Imaging and Therapy 2007;4:106-9. (in Chinese).
- Johnson R, Ding Y, Nagiah N, Monnet E, Tan W. Coaxially-structured fibres with tailored material properties for vascular graft implant. Mater Sci Eng C Mater Biol Appl 2019;97:1-11. [Crossref] [PubMed]
- Asenbaum U, Schoder M, Schwartz E, Langs G, Baltzer P, Wolf F, Prusa AM, Loewe C, Nolz R. Stent-graft surface movement after endovascular aneurysm repair: baseline parameters for prediction, and association with migration and stent-graft-related endoleaks. Eur Radiol 2019;29:6385-95. [Crossref] [PubMed]
- Wang G, Gao C, Xiao B, Zhang J, Jiang X, Wang Q, Guo J, Zhang D, Liu J, Xie Y, Shu C, Ding J. Research and clinical translation of trilayer stent-graft of expanded polytetrafluoroethylene for interventional treatment of aortic dissection. Regen Biomater 2022;9:rbac049. [Crossref] [PubMed]
- Lopez S, Tarmiz A, Rousseau H, Fournial G. Floating aortic thrombus: aortic trauma treated by heparin and delayed covered stent. Ann Vasc Surg 2011;25:984.e1-3. [Crossref] [PubMed]
- Gorog DA, Sweeny JM, Fuster V. Antiplatelet drug 'resistance'. Part 2: laboratory resistance to antiplatelet drugs-fact or artifact? Nat Rev Cardiol 2009;6:365-73. [Crossref] [PubMed]
- Choi PA, Parry PV, Bauer JS, Zusman BE, Panczykowski DM, Puccio AM, Okonkwo DO. Use of Aspirin and P2Y12 Response Assays in Detecting Reversal of Platelet Inhibition With Platelet Transfusion in Patients With Traumatic Brain Injury on Antiplatelet Therapy. Neurosurgery 2017;80:98-104. [Crossref] [PubMed]


