Evaluating the efficacy of balloon pulmonary angioplasty using quantitative analysis of SPECT pulmonary ventilation/perfusion scintigraphy in patients with chronic thromboembolic pulmonary hypertension
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by pulmonary thromboembolism (PTE) causing widespread obstruction of the pulmonary vascular bed. If untreated, progressive pulmonary vascular resistance (PVR) can lead to right-sided heart failure and worsen the patient’s prognosis (1). Currently, pulmonary endarterectomy (PEA) is the gold standard in treatment for CTEPH (2). Balloon pulmonary angioplasty (BPA) is a percutaneous pulmonary artery interventional therapy developed in recent years. Several studies have demonstrated that BPA can significantly improve the pulmonary hemodynamic state and prognosis in patients with CTEPH (3,4) and offers a promising treatment modality for patients with CTEPH who are unable to undergo PEA.
At present, BPA is indicated as an option in the following cases: patients who cannot undergo PEA due to distal vascular occlusion; patients with less severe pulmonary vascular disease but significantly reduced blood flow or those with comorbidities that significantly increase the risk of surgery; patients with persistent hypertension or recurrent pulmonary hypertension (PH) after PEA; and patients requiring salvage angioplasty following early failure of PEA (5).
In single-photon emission computed tomography (SPECT) pulmonary ventilation/perfusion (V/Q) scintigraphy, a noninvasive imaging technique that continues to be used in the diagnosis of CTEPH, the diagnosis is mainly based on mismatched V/Q scintigraphy due to impaired blood perfusion induced by thromboembolism (6). Furthermore, semi-quantitative measurement of the pulmonary perfusion defect percentage scores (PPDs%) can be used effectively for monitoring and evaluating treatment efficacy in CTEPH (7). There have been continued advances in the SPECT software in recent years. The image analysis software makes the quantitative assessment of pulmonary perfusion defects easier. Results can be generated more conveniently and the analysis can be more objective than semiquantitative measures of mismatch.
In this study, we explored the utility of quantitative analysis of V/Q scintigraphy in evaluating the efficacy of BPA in patients with CTEPH by comparing the percentage changes of preoperative and postoperative perfusion defects in patients who underwent BPA. Compared with the traditional semi-quantitative analysis based on visual analysis, quantitative analysis is more objective and reproducible, which is more conducive to the clinical application of V/Q imaging in the field of CTEPH. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1208/rc).
Methods
General information
We retrospectively collected V/Q scintigraphy data, clinical, and hemodynamic parameters from patients diagnosed with CTEPH in the China-Japan Friendship Hospital between April 2018 and September 2020. Diagnosis was as per the guidelines of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) for the diagnosis and treatment of pulmonary hypertension published in 2022 (8).
Exclusion criteria: (I) a history of pulmonary surgery or diseases complicated with vasculitis or pulmonary artery tumor; (II) the time interval between any V/Q imaging before or after surgery and the corresponding right heart catheterization was more than 1 week; (III) the right heart catheterization details or clinical data were incomplete.
A total of 67 eligible patients were screened from April 2018 to September 2020, including 13 patients who only underwent perfusion and lacked ventilation imaging, and three patients who had poor quality of ventilation or perfusion images due to technical problems that affected the accuracy of quantitative analysis. The final analysis included 51 patients and we analyzed 102 pre- and post-operative V/Q images. This included 23 males and 28 females, aged between 27–77 years, with an average age of 55.1±12.7 years.
All patients fulfilled the clinical diagnostic criteria for CTEPH [after at least 3 months of standardized anticoagulation therapy; the presence of chronic thrombosis confirmed by computed tomography (CT) pulmonary angiography, V/Q scintigraphy, or pulmonary angiography; right heart catheterization showed the mean pulmonary arterial pressure (mPAP) ≥25 mmHg at rest] (1). All patients underwent V/Q SPECT scintigraphy within one week prior to surgery, and pulmonary angiography was performed within 1–3 months following surgery. V/Q SPECT scintigraphy was repeated within one week before or after the repeat pulmonary angiography. Among these, 46 patients underwent BPA more than once. The duration of follow-up was 12.0±6.9 months.
General information about the patients and their pre- and post-operative clinical indicators were collected. Hemodynamic parameters were obtained using right heart catheterization. We divided patients into the residual pulmonary hypertension group and the non-residual pulmonary hypertension group based on the presence or absence of residual pulmonary hypertension (mPAP >30 mmHg) after surgery, respectively (9). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted with approval from the Ethics Committee of China-Japan Friendship Hospital (No. 2022-KY-085). Written informed consent was obtained from all participants.
Imaging method
Images were acquired using the dual-head gamma camera (Siemens Symbia T16 True point SPECT-CT; Siemens Medical Solutions, USA), which is equipped with a low-energy, high-resolution collimator. The energy peak was set at 140 keV with a window of ±10%. A disposable pulmonary ventilation introducer: 99mTc-technetium air ventilation device (VITA Medical Ltd., Australia), was used as the ventilation equipment. The V/Q scintigraphy was completed in one day.
Pulmonary ventilation tomography
An eluate of Technetium 99m pertechnetate (99mTcO4−) (with a concentration of >370 MBq/0.1 mL) was injected into the graphite tong pot of the technetium gas generator (VITA Medical Limited, Australia) and heated in the enclosed chamber filled with argon gas. Technetium was released when the 99mTcO4− evaporated. The mask was attached to the tube, and the patient was asked to inhale the technetium gas for 1 min. The patient was positioned lying on the back on the examination table, with arms over the head, so that the detector was placed as close to the chest as possible. The two detectors were rotated by 180°, and each detector acquired 32 projections, with 20 s for each projection. There were 64 projections in total. The acquisition matrix was 128×128, with a zoom factor of 1. The patient was instructed to breathe as normally as possible during the process of acquiring the images to minimize the influence of respiratory movements. The ordered subsets expectation maximization (OSEM) method was used to reconstruct the image (8 subsets, 2 iterations), and the coronal, sagittal, horizontal and 3D model images were obtained.
Pulmonary perfusion tomography
Before the start of the imaging procedure, the patient was instructed to inhale oxygen with a flow rate of 2–4 L/min and an inhalation period of 5–10 min. The patient was positioned on his/her back on the examination table, with arms raised over the head so that the detector was as close to the chest as possible. A total of 185–370 MBq 99mTc-labeled macroaggregated albumin (MAA) (Beijing Atomic Hi-Tech Co., Ltd., China; radiochemical purification ≥95%) was slowly administered intravenously. The acquisition parameters were the same as for pulmonary ventilation scintigraphy. Perfusion count-rate exceeded ventilation count-rate by a factor of 3 or more.
Low-dose CT scan
A low-dose CT scan was performed after the pulmonary perfusion tomography, with the patient remaining positioned on the same bed. The tube voltage was 110 kV, and the tube current was 25 mAs. The slice thickness was 5 mm. The parameters were collected, and the images were taken during breath-holding at the end of inspiration.
Image analysis
Quantitative analysis of images
All raw SPECT data were imported into the HERMES workstation (HERMES Medical Solutions AB, Sweden), where the V/Q ratio software provided in the system was used to reconstruct the perfusion and ventilation SPECT images, utilizing the low-dose CT images for attenuation correction. After the reconstruction, 10% of the maximum perfusion and ventilation imaging counts were selected as the threshold and set as the pulmonary boundary. After the radioactivity counts of perfusion and ventilation imaging images being normalized and corrected respectively, the respective radioactivity counts of each voxel were obtained, and then the radioactivity count ratio of perfusion and ventilation imaging images were obtained by dividing them.
The reconstructed perfusion/ventilation SPECT and low-dose CT images were imported into the system using the Hybrid 3D software. The boundary contours of the left and right lungs and the whole lung were automatically sketched on the lung CT to obtain the volume of interest (VOI). Areas exhibiting reverse mismatch on V/Q scintigraphy were manually delineated and removed. After processing, the software automatically generated the anatomic volume of the left and right lung based on CT images, the perfusion function volume of the left and right lung based on perfusion images, the ventilation function volume of the left and right lung based on ventilation images, and the volume of V/Q mismatch.
Semi-quantitative analysis of images
The V/Q scintigraphy was evaluated by two experienced nuclear medicine physicians who were blinded to the assessment. The number of lung segments with lung perfusion defects was recorded, and a semi-quantitative analysis of the pulmonary perfusion images was done using the Meyer method (10). The PPDs% was calculated. The normal functional volume percentage were as follows: 18% in the upper lobe of the right lung, 12% in the middle lobe of the right lung, and 25% in the lower lobe of the right lung; 13% in the intrinsic segment of the left upper lobe, 12% in the lingula segment of the left lung, and 20% in the lower lobe of the left lung. Each lobe was assigned a coefficient based on the severity of its perfusion defect. The degree of perfusion defect from mild to severe was rated as 0.75, 0.50, 0.25, and 0, respectively, with 1 representing completely normal pulmonary perfusion. The actual perfusion percentage for each lobe was obtained by multiplying the normal perfusion percentage and the coefficient of each lobe, and these were added to obtain the perfusion percentage for the whole lung. PPDs%, which reflected the severity of the pulmonary vascular perfusion defect, was calculated as 1 minus the pulmonary perfusion percentage.
Statistical analysis
IBM SPSS 19.0 software was used for statistical analysis. Quantitative data that conformed to the normal distribution were represented as , while those that did not conform to the normal distribution were represented as M (P25, P75). The correlation between V/Q scintigraphy quantitative parameters, semi-quantitative parameters, and the visual analysis of patients with CTEPH was conducted using Pearson’s correlation analysis. Paired t-test was used to compare the parameters before and after BPA in patients with CTEPH. Independent samples t-test was used to compare the pre- and post-operative quantitative PPDs% in the residual pulmonary hypertension group and the non-residual pulmonary hypertension group. A receiver operator characteristic (ROC) curve was used to calculate the area under the curve (AUC), and the cut-off values for the post-operative quantitative PPDs% and PPDs% against the pulmonary hypertension group were analyzed. The DeLong approach was used to test the difference in the AUC between the qualitative and semi-quantitative methods (MedCalc). Binary logistic regression model was used to analyze the related variables of residual pulmonary hypertension, and factors with a P value of <0.05 in the univariate test were included in the multivariate analysis to determine the final influencing factors of residual pulmonary hypertension. A P value of <0.05 was considered statistically significant, and all P value was a two-sided test.
Results
Clinical data
The sample for this study consisted of a total of 51 patients diagnosed with CTEPH who underwent BPA between April 2018 and September 2020 and the patient selection flowchart is shown in Figure 1. There were 23 males and 28 females. The patients were aged 27 to 77 years, with an average age of 55.1±12.7 years. The average BMI was 24.2±3.4 kg/m2, and the average 6-minute walking distance (6MWD) was 379±99 m. There were 7 patients with grade I cardiac function, 21 patients with grade II, 19 patients with grade III, and 4 patients with grade IV cardiac function as per the criteria of the New York Heart Association (NYHA) Functional Classification.
Among the 51 patients included in the study, 147 BPA treatments were performed and a total of 588 pulmonary arteries were treated, with an average of 4.0 pulmonary arteries treated per operation. Among them, 46 patients had BPA ≥2 times and were followed up for 12.0±6.9 months. All patients with BPA were treated with anticoagulant therapy after the surgery. Among them, hemoptysis occurred in 8 cases (5.4%) after BPA, and reperfusion pulmonary edema (RPE) occurred in 4 cases (2.9%).
Correlation analysis of the quantitative and semi-quantitative results of pulmonary V/Q scintigraphy
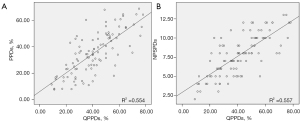
Fifty-one patients underwent a total of 102 V/Q scintigraphy scans before and after surgery. The quantitative PPDs% were (41.2±16.6)%, and the semi-quantitative PPDs% were (35.4±16.0)%. The number of pulmonary segments with perfusion defects (NPSPDs) was 7.8±2.6. Correlation analysis showed a positive relationship between quantitative PPDs and semi-quantitative PPDs (r=0.744, P<0.001) and between quantitative PPDs and NPSPDs (r=0.746, P<0.001), as shown in Figure 2.
Correlation analysis of quantitative indexes of pulmonary V/Q scintigraphy and hemodynamic and clinical parameters
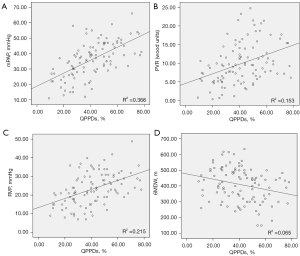
Based on the 102 V/Q scintigraphy data, right heart catheterization, and clinical parameters, the quantitative PPDs% was positively correlated with mPAP, PVR, and right ventricular pressure (RVP) (r=0.605, 0.391, and 0.464, respectively, all P<0.001) and negatively correlated with 6MWD (r=−0.254, P=0.010), as shown in Figure 3.
Comparison of preoperative and postoperative V/Q scintigraphy, clinical parameters, and hemodynamic parameters in patients with CTEPH
As seen in Table 1, the preoperative NPSPDs, PPDs%, and quantitative PPDs% were all decreased after the BPA (all P<0.001). Additionally, the preoperative value of 6MWD was 379±99 m, which rose to 440±108 m after the BPA (t=−5.309, P<0.001).
Table 1
| Item | Before BPA | After BPA | t/Z value | P value |
|---|---|---|---|---|
| NPSPDs (n) | 9.0±2.5 | 6.5±2.2 | 8.383 | <0.001 |
| PPDs% | 43.1±15.4 | 27.6±12.5 | 7.898 | <0.001 |
| Quantitative PPDs (%) | 49.0±15.6 | 33.5±13.9 | 11.249 | <0.001 |
| 6MWD (m) | 379±99 | 440±108 | −5.309 | <0.001 |
| NT-proBNP (pg/mL) | 503 [120–1,002] | 226 [78–700] | −4.307 | <0.001 |
| Mixed venous oxygen saturation (%) | 64.2±5.7 | 66.8±5.9 | −3.644 | 0.001 |
| Serum creatinine level (μmol/L) | 77.1±16.2 | 77.1±15.2 | −0.058 | 0.954 |
| D-dimer (ng/mL) | 0.55±0.64 | 0.42±0.45 | 1.79 | 0.08 |
Data are means ± SD or median [interquartile range]. V/Q, ventilation/perfusion; CTEPH, chronic thromboembolic pulmonary hypertension; BPA, balloon pulmonary angioplasty; NPSPDs, the number of pulmonary segments with perfusion defects; PPDs%, pulmonary perfusion defect percentage scores; 6MWD, 6-min walking distance; NT-proBNP, N-terminal pro B-type natriuretic peptide.
As shown in Table 2, all the pulmonary hemodynamic parameters of patients with CTEPH, including mPAP, PVR, and RVP after BPA, were significantly improved (all P<0.001).
Table 2
| Item | Before BPA | After BPA | t/Z value | P value |
|---|---|---|---|---|
| mPAP (mmHg) | 42.2±8.6 | 30.2±11.1 | 8.198 | <0.001 |
| PVR (Wood) | 12.3±5.0 | 7.5±4.9 | 7.017 | <0.001 |
| RVP (mmHg) | 26.8±8.0 | 19.9±8.1 | 6.499 | <0.001 |
| RAP (mmHg) | 4.5±2.7 | 3.9±2.4 | 1.867 | 0.068 |
| PAWP (mmHg) | 9.1±2.8 | 9.5±2.6 | −0.967 | 0.338 |
Data are mean ± standard deviation. 1 mmHg =0.133 kPa, 1 Wood =80 dns·s·cm−5. BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; RVP, right ventricular pressure; RAP, right atrial pressure; PAWP, pulmonary arteriole wedge pressure.
Comparison of the quantitative parameters of V/Q scintigraphy patients with or without postoperative residual pulmonary hypertension
As shown in Table 3, out of the 51 patients with CTEPH, 27 had no residual pulmonary hypertension while the other 24 had residual pulmonary hypertension. The pre- and postoperative quantitative PPDs% in the residual pulmonary hypertension group were higher than those in the non-residual pulmonary hypertension group (t=2.599, P=0.012; t=4.647, P<0.001, respectively).
Table 3
| Group | mPAP ≤30 mmHg (n=27) | mPAP >30 mmHg (n=24) | t/Z value | P value |
|---|---|---|---|---|
| Age (years) | 51.9±14.6 | 58.8±9.2 | 2.031 | 0.048 |
| Gender (male/female) | 14/13 | 9/15 | 0.400 | |
| Quantitative PPDs% pre-surgery | 44.0±13.8 | 54.7±15.7 | 2.599 | 0.012 |
| Quantitative PPDs% post-surgery | 26.3±11.0 | 41.5±12.5 | 4.647 | <0.001 |
Data are means ± SD or numbers of subjects. PPDs%, quantitative pulmonary perfusion defect percentage scores; mPAP, mean pulmonary artery pressure.
Difference in diagnostic efficacy of quantitative PPDs% and PPDs% in residual pulmonary hypertension
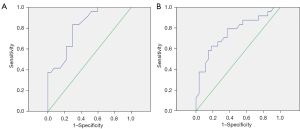
The cut-off values for the residual pulmonary hypertension group and the non-residual pulmonary hypertension group were 28.5% and 31.0%, respectively. The sensitivity and specificity for the evaluation of quantitative PPDs% in residual pulmonary hypertension was 83.3% and 70.4%, respectively. The sensitivity and specificity for the evaluation of PPDs% in residual pulmonary hypertension was 62.5% and 81.5% respectively. There was no significant difference in the diagnostic efficacy between the quantitative PPDs% and PPDs% analyses (DeLong Z value =0.799, P=0.424; see Table 4 and Figure 4).
Table 4
| Item | Sensitivity (%) | Specificity (%) | Cut-off value | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| QPPDs% | 83.3 | 70.4 | 28.5 | 0.812 (0.697–0.927) | <0.001 |
| PPDs% | 62.5 | 81.5 | 31.0 | 0.754 (0.618–0.890) | 0.002 |
There was no significant difference in the diagnostic efficacy between the quantitative PPDs% and PPDs% analyses (DeLong Z value =0.799, P=0.424). QPPDs%, quantitative pulmonary perfusion defect percentage scores; PPDs%, pulmonary perfusion defect percentage scores; ROC, receiver operating characteristic; AUC, areas under the curve; CI, confidence interval.
Analysis of factors influencing residual pulmonary hypertension after BPA
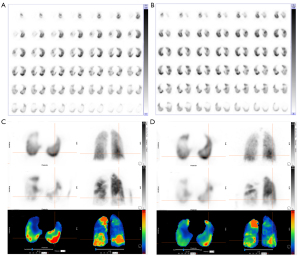
We analyzed gender, age, preoperative QPPDs%, BMI, 6WMD, cardiac function grade, mPAP, PVR, RVP, RAP, and other indicators using a single factor analysis. The results showed that preoperative QPPDs% (OR =1.052, 95% CI: 1.008–1.098, P=0.019), mPAP (OR =1.092, 95% CI: 1.010–1.181, P=0.028), and RVP (OR =1.103, 95% CI: 1.011–1.203, P=0.027) were factors influencing residual pulmonary hypertension after BPA surgery. Multivariate regression analysis showed that QPPDs% was not a significant factor for residual pulmonary hypertension (OR =1.043, 95% CI: 0.995–1.094, P=0.079). See Figure 5 for typical patient images.
Discussion
Due to acute pulmonary embolism or repeated pulmonary artery thrombosis that cannot be completely dissolved, chronic inflammation, and thickening of the pulmonary artery intima, CTEPH eventually develops into chronic pulmonary embolism, culminating in pulmonary hypertension. The key findings of our study were as follows: (I) the quantitative analysis index QPPDs% of V/Q imaging correlated well with the traditional semi-quantitative analysis for CTEPH image analysis, and quantitative analysis demonstrated obvious advantages because of its objectivity, simplicity of use, and high repeatability; (II) quantitative analysis index QPPDs% adequately reflected the pulmonary artery pressure and clinical status of patients, and preoperative QPPDs% was found to be an independent risk factor for postoperative residual pulmonary hypertension. Quantitative analysis of V/Q imaging serves as a valuable indicator for predicting the prognosis and assessing the postoperative efficacy of BPA in patients with CTEPH. Lack of timely diagnosis or treatment can result in delays, affect the prognosis, and eventually endanger life. Chronic thrombosis and increased pulmonary artery pressure lead to an imbalanced pulmonary ventilation and blood perfusion ratio, which is reflected in V/Q scintigraphy.
The guidelines for the diagnosis and treatment of pulmonary hypertension published by the ESC and the ERS in 2022 (8) recommend pulmonary V/Q scintigraphy as the preferred test for the diagnosis of CTEPH. The diagnosis of CTEPH can be safely ruled out with a negative V/Q scintigraphy result, with grade I recommendation and class C evidence. However, the conventional V/Q scintigraphy based on visual evaluation cannot be used for quantitative analysis as it lacks quantitative analysis indicators that can objectively reflect the severity of CTEPH. Furthermore, there are additional limitations in that it is inconvenient and challenging to standardize this method, making it difficult to establish an objective measure of the severity of CTEPH.
The quantitative PPDs% measured by SPECT/CT quantitative software can make up for these limitations and provide an effective method for quantitatively assessing the extent of disease involvement and severity of CTEPH in patients. The main advantages of quantitative analysis are its simplicity of operation, objectivity, and strong repeatability. Besides, subjective aspects such as the experience and personal ability of viewers have little impact on the results, giving it obvious advantages over semi-quantitative analyses.
In this study, we found that the quantitative analysis of PPDs% based on the software correlated well with the traditional semi-quantitative analysis of PPDs% and reflected pre- and post-operative hemodynamics and clinical status of patients with CTEPH, thereby demonstrating the feasibility of its use to analyze images in patients with CTEPH. The quantitative analysis indexes were more objective, reproducible, and not constrained by the professional competence of the reader, all of which contributed to their reliability. We found that the quantitative PPDs% was positively correlated with the hemodynamic indicators mPAP, PVR, and RVP and negatively correlated with 6MWD. Consistent with earlier research in China and elsewhere (11-13), the results showed that the quantitative analysis of V/Q scintigraphy could reflect the hemodynamics and clinical status of patients with CTEPH. Ultimately, patients will certainly benefit from the entire treatment process as the use of this non-invasive imaging means can reflect their postoperative hemodynamic status and thereby minimize the use of invasive pulmonary angiography for routine reviews.
Drugs and surgery are currently the mainstays of treatment for CTEPH, with surgery being the only cure for the condition. The most common surgical treatments for CTEPH primarily include PEA, BPA, and lung transplantation or combined heart-lung transplantation. PEA surgery remains the preferred therapeutic choice for CTEPH at present. BPA is a percutaneous pulmonary artery interventional therapy developed in recent years, which can be considered for certain categories of patients such as some patients who are not eligible for PEA surgery or residual pulmonary hypertension after PEA surgery (13).
Currently, computed tomography pulmonary angiography (CTPA) and V/Q scintigraphy are the popular non-invasive imaging methods for evaluating the efficacy of BPA in patients with CTEPH. CTPA is a non-invasive approach with high resolution for accurately evaluating the patency of proximal pulmonary blood vessels. However, CTPA has a high radiation dosage and is therefore not suitable for repeated use. Due to its restricted capability in diagnosing peripheral small blood vessels, CTPA can often yield false negative results. Additionally, patients with a history of anaphylaxis, triggered by any allergen, face an elevated risk of hypersensitivity-type reactions to iodinated IV contrast.
In this study, the postoperative mPAP, PVR, RVP, N-terminal pro B-type natriuretic peptide (NT-proBNP), and mixed venous oxygen saturation substantially decreased compared with the preoperative levels, while the 6MWD was significantly higher than that before surgery. Our findings that BPA definitively improved the hemodynamic parameters and exercise tolerance in patients with CTEPH are in line with those of several previous studies (4,14-16).
A study conducted in 2021 revealed that the semi-quantitative PPDs% on V/Q scintigraphy correlated with hemodynamic measures after PEA. Further, preoperative PPDs% was identified as one of the main risk factors for residual pulmonary hypertension following PEA (7). Hashimoto et al. found that functional %volume of the lung calculated from the lung perfusion SPECT (FVL-LPSPECT) based on SPECT pulmonary perfusion imaging was significantly improved after BPA surgery, which, like the hemodynamic index, mPAP, and the clinical parameter, 6MWD, can reflect the postoperative improvement of patients (16). The authors of this study also found that the postoperative NPSPDs, PPDs%, and quantitative PPDs% after BPA measured using V/Q scintigraphy decreased as compared with the preoperative ones, indicating that the semi-quantitative and quantitative parameters of V/Q scintigraphy both could effectively reflect the improvements that BPA surgery had on pulmonary blood perfusion. Our result pertaining to the evaluation of the postoperative efficacy of BPA is consistent with the findings of Wang et al. (14). On the basis of traditional semi-quantitative analysis, we found that among the hemodynamic measures, the quantitative PPDs% was predictive of post-BPA.
In this study, we further found that among 51 patients with CTEPH, 24 had residual pulmonary hypertension post-surgery, accounting for about 47.1% (24/51 cases), which is higher than the 25% incidence of postoperative residual pulmonary hypertension in patients with CTEPH reported in literature (17). A probable explanation is that all of the 51 patients with CTEPH included in this study were patients who underwent BPA surgery because they were found unsuitable for PEA. Most patients had distal vascular occlusion or were patients who have a low risk/benefit ratio for PEA treatment after evaluation, such as those with less severe pulmonary vascular disease but with significantly reduced blood flow or those with comorbidities that significantly increase the risk of surgery. The clinical profile of these patients is typically more complicated than that of patients who are candidates for PEA, resulting in a higher incidence of residual pulmonary hypertension (18). In one study, it was found that when the CT pulmonary angiography after PEA showed ≤2 residual pulmonary segments with perfusion defects, the proportion of residual pulmonary hypertension was significantly reduced (19).
In contrast to CTPA, which is premised on documenting obstruction of (larger) pulmonary vessels, V/Q scintigraphy is better suited to the identification of obstructive lesions in smaller, more peripheral blood vessels. In this study, the proportion of patients with preoperative quantitative PPDs% in the residual pulmonary hypertension group was significantly higher than that of the non-residual pulmonary hypertension group, indicating that the preoperative quantitative PPDs% could be a useful predictor of postoperative outcomes. As the preoperative quantitative PPDs% increases, the incidence of residual pulmonary hypertension after surgery is higher. In this study, the postoperative quantitative PPDs% in the residual pulmonary hypertension group was higher than that of the non-residual pulmonary hypertension group, indicating that the quantitative PPDs% in V/Q scintigraphy can objectively and non-invasively reflect the hemodynamic status in patients with CTEPH after BPA or can be used as an effective reference index to evaluate the efficacy of surgery.
There are some limitations to this study. This was a single-center retrospective study with a relatively small sample size. Further, multicenter studies with larger samples and prospective research data are needed to verify the utility of V/Q scintigraphy quantitative analysis in evaluating the efficacy of CTEPH in patients. Further studies are needed to explore the difference between quantitative analysis and conventional methods of analysis in the diagnosis of CTEPH, and to further validate its clinical utility. In addition, the measurement of quantitative analysis parameters of V/Q scintigraphy in this study was still at the level of the whole lungs and pulmonary lobes. In order to better address clinical needs, additional research is needed in the future to realize the quantitative analysis of V/Q scintigraphy at the lung segment level.
Conclusions
In this study, we found that it was possible to quantitatively assess the percentage of pulmonary V/Q mismatched defects in patients with CTEPH using SPECT pulmonary V/Q scintigraphy, and this quantification corresponded with the results of conventional visual analysis. Further, it adequately reflected the pulmonary artery pressure and clinical status in patients with CTEPH, indicating its definite utility in predicting residual pulmonary hypertension and evaluating the postoperative efficacy of BPA in patients with CTEPH.
Acknowledgments
The authors would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1208/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1208/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted with approval from the Ethics Committee of China-Japan Friendship Hospital (No. 2022-KY-085). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nossent EJ, Meijboom LJ, Bogaard HJ, Klok FA. Chronic thromboembolic pulmonary hypertension anno 2021. Curr Opin Cardiol 2021;36:711-9. [Crossref] [PubMed]
- Ruaro B, Confalonieri P, Caforio G, Baratella E, Pozzan R, Tavano S, Bozzi C, Lerda S, Geri P, Biolo M, Cortale M, Confalonieri M, Salton F. Chronic Thromboembolic Pulmonary Hypertension: An Observational Study. Medicina (Kaunas) 2022;58:1094. [Crossref] [PubMed]
- Mahmud E, Patel M, Ang L, Poch D. Advances in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Pulm Circ 2021;11:20458940211007385. [Crossref] [PubMed]
- Jin Q, Luo Q, Yang T, Zeng Q, Yu X, Yan L, Zhang Y, Zhao Q, Ma X, An C, Xiong C, Zhao Z, Liu Z. Improved hemodynamics and cardiopulmonary function in patients with inoperable chronic thromboembolic pulmonary hypertension after balloon pulmonary angioplasty. Respir Res 2019;20:250. [Crossref] [PubMed]
- Collaud S, Brenot P, Mercier O, Fadel E. Rescue balloon pulmonary angioplasty for early failure of pulmonary endarterectomy: The earlier the better? Int J Cardiol 2016;222:39-40. [Crossref] [PubMed]
- Bajc M, Schümichen C, Grüning T, Lindqvist A, Le Roux PY, Alatri A, Bauer RW, Dilic M, Neilly B, Verberne HJ, Delgado Bolton RC, Jonson B. EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond. Eur J Nucl Med Mol Imaging 2019;46:2429-51. [Crossref] [PubMed]
- Wang L, Wang M, Zhang ZY, Zhang HL, Fang W. Pulmonary ventilation/perfusion scintigraphy in assessing residual pulmonary hypertension after pulmonary endarterectomy. Chinese Journal of Nuclear Medicine and Molecular Imaging 2021;41:257-61.
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023;61:2200879. [Crossref] [PubMed]
- Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation 2016;133:1761-71. [Crossref] [PubMed]
- Meyer G, Collignon MA, Guinet F, Jeffrey AA, Barritault L, Sors H. Comparison of perfusion lung scanning and angiography in the estimation of vascular obstruction in acute pulmonary embolism. Eur J Nucl Med 1990;17:315-9. [Crossref] [PubMed]
- Ma RZ, Han PP, Tao XC, Li H, Wang L, Zhai ZG, Fu LP. A Feasibility Study on Using Single-Photon Emission Computed Tomography Pulmonary Perfusion/Ventilation Imaging for the Diagnosis of Chronic Thromboembolic Pulmonary Hypertension and Patient Risk Assessment. Int J Gen Med 2021;14:8029-38. [Crossref] [PubMed]
- Özgüven S, Kesim S, Öksüzoğlu K, Yanartaş M, Taş S, Şen F, Öneş T, İnanır S, Turoğlu HT, Mutlu B, Erdil TY, Yıldızeli B. Correlation Between Perfusion Abnormalities Extent in Ventilation/Perfusion SPECT/CT with Hemodynamic Parameters in Patients with Chronic Thromboembolic Pulmonary Hypertension. Mol Imaging Radionucl Ther 2021;30:28-33. [Crossref] [PubMed]
- Zhang L, Bai Y, Yan P, He T, Liu B, Wu S, Qian Z, Li C, Cao Y, Zhang M. Balloon pulmonary angioplasty vs. pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. Heart Fail Rev 2021;26:897-917. [Crossref] [PubMed]
- Wang L, Han X, Wang M, Ma X, Zhang H, Yan C, Fang W. Ventilation/perfusion imaging predicts response to balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Ann Nucl Med 2022;36:515-22. [Crossref] [PubMed]
- Brenot P, Jaïs X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, Mercier O, Fabre D, Parent F, Jevnikar M, Montani D, Savale L, Sitbon O, Fadel E, Humbert M, Simonneau G. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1802095. [Crossref] [PubMed]
- Hashimoto H, Oka T, Nakanishi R, Mizumura S, Dobashi S, Hashimoto Y, Okamura Y, Ota K, Ikeda T. Evaluation of balloon pulmonary angioplasty using lung perfusion SPECT in patients with chronic thromboembolic pulmonary hypertension. J Nucl Cardiol 2022;29:3392-400. [Crossref] [PubMed]
- Hsieh WC, Jansa P, Huang WC, Nižnanský M, Omara M, Lindner J. Residual pulmonary hypertension after pulmonary endarterectomy: A meta-analysis. J Thorac Cardiovasc Surg 2018;156:1275-87. [Crossref] [PubMed]
- Braams NJ, Ruigrok D, Schokker MGM, Padervinskiene L, de Man FS, Marcus JT, Lely RJ, Beijk MAM, Klok FA, Huisman MV, Nossent EJ, Vonk Noordegraaf A, Symersky P, Bogaard HJ, Meijboom LJ. Pulmonary vascular imaging characteristics after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2020;39:248-56. [Crossref] [PubMed]
- Zhu JD, Wang HP, Deng L, et al. The influence of the residual occluded pulmonary segments on the surgical efficacy of pulmonary thromboendarterectomy. Chinese Circulation Journal 2019;34:790-5.