
Left ventricular function assessment including or excluding trabeculations in left ventricular non-compaction, a preliminary case-control cardiac magnetic resonance study
Introduction
Echocardiography is used as a first line examination in suspected cases of left ventricular non-compaction (LVNC), and often needs confirmation with cardiac magnetic resonance (CMR) imaging. These two techniques mainly rely on the basis of morphological diagnostic criteria that have been published (1) and are generally accepted in clinical use.
It is acknowledged that the LVNC phenomenon induces morphological myocardial alterations such as the persistence of hypertrabeculations that may have deleterious consequences including intertrabecular recess thrombus formation (2-4). This occurrence is favored by the absence of motion of the trabecular meshwork, which could distinguish LVNC from hypertrabeculations, a well-known phenotype encountered in several structural heart conditions and in a various amount of healthy subjects (5). Using only standard morphologic CMR imaging criteria of trabeculated, non-compact (NC) and compacted myocardium (C) with a NC/C ratio of >2.3 (Petersen criterion), overdiagnosis of LVNC has been suggested (5). To solve the CMR ambiguities using this parameter in the diagnosis of LVNC, several other measurements have been proposed (6), including the trabeculated left ventricular (LV) mass (Jacquier criterion), the trabeculated LV volume (Choi criterion), and the trabeculated LV mass and distribution (Grothoff criterion).
We tried to elucidate whether the LVNC diagnosis should be facilitated by hypothesizing that significant differences could be exhibited when measuring concurrently LV volume, mass and ejection fraction (LVEF) of the compacted myocardium on one hand and of the overall myocardium on the other hand, i.e., without and with trabeculation inclusion in the LV mass. In LVNC, the volume of non-compact fibers is expected not to change all along the cardiac cycle with a passive fiber motion, whereas in simply hypertrabeculated individuals the volume of trabeculations should decrease due to active shortening during systole. To our knowledge, no such study has been previously published upon this specific concern.
Following this hypothesis, we made the assumption that including the trabecular meshwork in the LV mass would improve LVEF in LVNC patients, whereas it would decrease LVEF in hypertrabeculated myocardium because in this case trabeculations keep contractile motion.
The primary endpoint was to compare LV volumes, LV shape (eccentricity index, EI), ejection fraction and mass in subjects above and below the classical cutoff NC/C ratio of 2.3 to better classify them as LVNC or hypertrabeculated. The secondary objective was to compare outcomes of the two groups according to the LVEF patterns. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1274/rc).
Methods
Patient selection
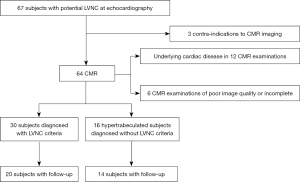
From the echocardiographic registry of the Bichat University Hospital, all patients referred for hypertrabeculations are classified as LVNC or hypertrabeculated. Between March 2011 and October 2018, 67 consecutive patients underwent CMR to precise echocardiographic findings. Thirty consecutive patients newly diagnosed as having LVNC with normal LVEF on the basis of current clinical and echocardiographic criteria (7) were selected from our database based on LVNC diagnosis (including reports and image files). LVNC diagnosis included a NC/C ratio >2.3 in diastole, and trabeculations involving more than 20% of the LV.
In those cases, LVNC diagnosis was confirmed on the basis of the following criteria: electrocardiographic abnormalities, electrophysiology testing, positive family history of cardiomyopathy and sudden cardiac death, positive genotype, documentation of a similar appearance in first-degree relatives, associated neuromuscular disorders. Exclusion criteria were poor CMR image quality, and history of active or prior cardiac disease.
The CMR findings from this LVNC group were compared to those of a consecutive group of 16 individuals matched for sex and age (±5 years of difference allowed) with existing trabeculations, whose range did not clearly fulfill the previously mentioned CMR criteria (NC/C ratio between 1.8 and 2.2, trabeculations involving 10% to 17% of LV) and who were selected as controls in the absence of other structural heart disease (Table 1, Figure 1). None of these controls had prior or familial history of cardiac disease or cardiovascular risk factors.
Table 1
| Variables | LVNC 30 patients | Hypertrabeculations 16 subjects | P |
|---|---|---|---|
| Age (years), median [IQR] | 41.3 [27.5–52.5] | 45.6 [39–53.5] | 0.062 |
| Male gender, n [%] | 18 [60] | 10 [63] | >0.99 |
| Indications for CMR, n [%] | |||
| Familial/genetic history of LVNC | 6 [20] | 0 | 0.021 |
| Hypertrabeculations characterization (healthy athletes, arterial hypertension) |
17 [57] | 11 [69] | 0.528 |
| LV hypertrophy | 6 [20] | 5 [31] | >0.99 |
| Associated cardiac indications, n [%] | |||
| Cardiac failure (NYHA III or IV) | 0 | 0 | >0.99 |
| Altered ECG, n [%] | |||
| Bundle branch block | 3 [10] | 1 [6] | >0.99 |
| Biomarkers, median [IQR] | |||
| Troponin T (ng/mL) | 51 [28–116] | 56 [32–114] | 0.942 |
| CPK (mg/dL) | 4 [3–139] | 4 [2–156] | >0.99 |
| NT-proBNP (pg/mL] | 274 [87–655] | 289 [94–721] | 0.979 |
| Echocardiographic LVEF [%], median [IQR] | 52 [50–73] | 56 [50–78] | 0.667 |
| LGE, n [%] | 6 [20] | 0 | 0.021 |
Chi-square test result for qualitative variables, other Ps are of Student’s t-test or Mann-Whitney U test result. LVNC, left ventricular noncompaction; IQR, interquartile range; CMR, cardiac magnetic resonance; LV, left ventricle; ECG, electrocardiogram; CPK, creatine phosphokinase; NT-proBNP, N-terminal pro brain natriuretic peptide; LVEF, left ventricle ejection fraction; LGE, late gadolinium enhancement.
This case-control study was designed as a retrospective analysis of patients treated at a single institution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Bichat University Hospital. Informed consent was waived with the approval of the institutional review board due to the retrospective nature of the study.
2D transthoracic echocardiography (TTE)
Comprehensive TTE was performed by two investigators with 3.5-MHz transducers equipped with harmonic imaging (Vivid 7, General Electric, Buc, France). Images were acquired in the long-axis parasternal view, the three short axis views (basal, mid, apical), and the 2-, 3- and 4-chamber apical views. These views were used to visualize all 17 segments according to the American Heart Association (AHA) recommendations. Data were analyzed offline on digitally stored images.
Magnetic resonance (MR) acquisition process and reading criteria
MR examinations were performed using a 1.5T GE MR 450 or a 3.0T GE Signa HDxt Discovery system (GE Healthcare, Waukesha, Wisconsin). MR images were acquired using a dedicated cardiac phased-array coil. All patients were examined following the same standard ECG-gated MR protocol consisting of cine steady-state free precession (SSFP) imaging for cardiac function, sequential dynamic injection during contrast medium injection to study early perfusion and LGE imaging for myocardial fibrosis.
Standard cine CMR images were acquired in three planes covering the whole left ventricle, using short-axis, long-axis and 4-chamber views before injecting the gadolinium with typical SSFP sequence parameters according to the MR system: repetition time between 3.1 and 3.4 ms; echo time between 1.3 and 1.5 ms; flip angle 45°; slice thickness 8 mm; field of view 320–350 mm; matrix size 256×256 pixels; 30 phases per heartbeat. T1 and T2 mapping sequences were not available on our MR systems during the inclusion period except in the last 3 patients.
LV volumetrics, measurements of myocardial thickness in trabeculae and compact myocardium were done in accordance with the standardized myocardial segmentation, as recommended by professional associations (8).
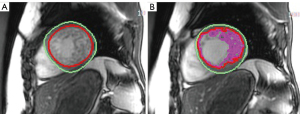
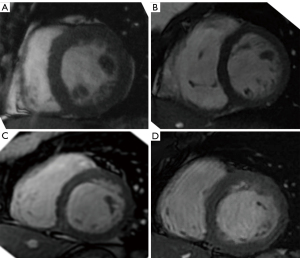
Functional CMR examination was assessed using a stack of standard ECG-gated SSFP short axis images covering the LV from base to apex. Quantitative analysis of CMR images, including LV function and mass was conducted by semi-automatic contour detection using certified CMR analysis software (cmr42, Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada) and region growing algorithms, manually corrected by two observers experienced in a robust contour tracing protocol (8), who traced two successive endocardial (presence then absence of trabeculation inclusion) and one epicardial contours (Figure 2).
Cardiac output was calculated on TTE as on CMR multiplicating the stroke volume by the heart rate, and compared to the cardiac output on TTE using the continuity equation.
LV end-diastolic EI was defined as the ratio of the anterior-inferior and septal-posterolateral cavity dimensions at the mid-ventricular level, i.e. of the long axis end diastolic diameter divided by the diameter of perpendicular line (compact to compact myocardium).
The presence, absence or partial agenesis of the papillar muscles was assessed qualitatively (Figure 3). LGE was recorded as present or absent whatever its pattern and localization.
Statistical analysis
In the field of this observational study, no attempt was made to calculate the sample size necessary to exhibit any statistical differences. All data are presented as mean ± standard deviation. Statistical analysis was performed using GraphPad Prism (La Jolla, CA, USA, Version 8.2.1). Functional parameters from matched anatomic images were compared using two-sided Student’s paired t-test. Pearson product moment correlation coefficients were calculated. Bland-Altman analysis determined the inter- and intra-reader reproducibility of functional data, as well as the agreement between automatic and manual tracing for LVEF measurements. An intra-class correlation coefficient (ICC) value of <0.5 was considered poor reliability, 0.5–0.75 moderate reliability, 0.75–0.9 good reliability and >0.9 excellent reliability (9). Statistical significance was considered at P<0.05.
Follow-up
A follow-up by phone call with the physician, echocardiography and/or CMR was available at 2 years in 20 LVNC patients and 14 controls.
Results
All patients and controls were in sinus rhythm. Patients with constant arrhythmia (atrial fibrillation or more than five premature beats per minute), were not considered for inclusion. Six LVNC patients had a positive genotype. A bundle branch block was present in 3 LVNC patients and one control (Table 1). Clinical and biomarker data were unable to distinguish between subjects displaying LVNC and hypertrabeculations (Table 1).
On CMR, 6 patients displayed LGE, all of them within the trabecular meshwork at one or two-segment level. Patients undergoing T1 and T2 mapping had normal T1 and T2 values. All subjects had normal “compact” LVEF on echocardiography as well as on CMR (Table 2). Main CMR functional patterns of LVNC were an increased overall end-diastolic mass when trabeculations were included, and a decreased end-diastolic mass when endocardial borders were limited to the compact myocardium (P<0.0001 for all). These differences were also significant for the systolic mass (P=0.0018); on the opposite, the differences were not as significant in controls (P=0.032). LVEF (Table 2) increased by 9.8%±1.6% in LVNC when trabeculae were included in the endocardial contours whereas it decreased by 10.9%±1.4% in hypertrabeculated controls (P<0.0001).
Table 2
| Parameters | LVNC | Controls | P | |||||
|---|---|---|---|---|---|---|---|---|
| Overall myocardium including trabeculae | Compact myocardium | Overall myocardium including trabeculae | Compact myocardium | Overall myocardium including trabeculae | Compact myocardium | |||
| LVEF (%) | 56.8±11.9 | 51.7±13.8 | 50.6±12.9 | 56.8±11.2 | 0.123 | 0.221 | ||
| End diastolic mass (g/m²) | 106.1±25.3 | 63.9±19.6 | 99.8±22.6 | 53.7±15.1 | 0.424 | 0.084 | ||
| End systolic mass (g/m²) | 92.7±24.2 | 64.9±19.7 | 74.5±14.9 | 57.9±14.1 | <0.011 | 0.221 | ||
| End diastolic LV volume (mL/m²) | 62.3±16.9 | 95.1±18.5 | 46.8±19.5 | 97.6±20.7 | 0.009 | 0.679 | ||
| End systolic LV volume (mL/m²) | 26.9±15.6 | 45.9±16.7 | 23.1±16.8 | 42.1±20.4 | 0.521 | 0.583 | ||
Data are presented as mean ± standard deviation. Comparisons were made with a two-tailed, paired Student’s t-test. P<0.05 was considered significant. LVEF, left ventricle ejection fraction; LV, left ventricle; LVNC, left ventricular noncompaction.
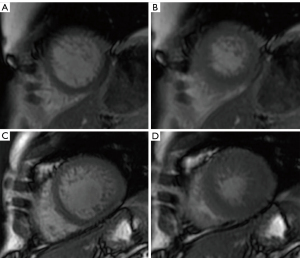
Among all the other parameters studied, LVNC patients and controls displayed the following significant differences: LVEF of the overall myocardium including trabeculae (i.e. compact + non-compact myocardium) (P=0.03) and LV diastolic volumes (P=0.0035). Considering the variation of LV volumes and mass in diastole and systole, the non-compact mass decrease in LVNC differed significantly (P<0.0001) from that of controls (Table 2, Figure 4).
Cardiac output measurements were not statistically different according to the two TTE calculation methods and CMR values excluding trabeculations (5.6±1.7, 5.2±1.5 and 5.5±1.2 L/mn respectively, P>0.05), but all displayed significant differences with CMR values including trabeculations (3.8±1.3 L/mn, P<0.001).
Qualitative assessment of papillar muscles was as follows: identifiable and normal in 2 LVNC patients, both atrophic in 15 patients, a single one atrophic (posterior inferior) in 6 patients, and absent in 7 patients; identifiable and normal in 11 hypertrabeculated controls, both atrophic in 2 controls and a single one atrophic (posterior inferior) in 3 other controls. The differences between the two populations were significant (P=0.0003). The EI was 0.69±0.08 in LVNC and 0.55±0.08 in hypertrabeculated controls (P<0.0001).
LVEF measurements including or excluding trabeculations demonstrated strong intra- and inter-observer reproducibility. Bland–Altman analysis demonstrated high intraobserver agreement for LVEF in compact myocardium (mean bias =1.8%), with a high Pearson correlation (r=0.88, P=0.001). Variability was modestly higher in assessing LV mass and LVEF of the overall myocardium (mean bias =3.1%), though the correlation remained strong (r=0.81, P=0.01). LVEF showed good agreement between different operators (Compact: mean bias =1.3%; overall mean bias =4.1%) with strong correlation (r=0.79, P=0.01).
Manual vs. automatic tracing measurements
Reproducibility of volume and mass measurements was good to excellent (ICC >0.75) for the two groups of subjects.
The bias between the two methods was –1.09±1.22 g/m2 for the trabeculated mass, and –0.86±1.68 g/m2 for the compacted mass.
Follow-up
The outcomes of the two groups were not statistically different. Follow-up echocardiography and/or CMR (Table 3) showed cardiac function worsening in 35% of LVNC patients (among them 5% needing cardiac transplantation) vs. 7% of hypertrabeculated controls. Patients with cardiac function worsening did not display any significant differences (LVEF with or without trabeculation inclusion, mass, geometry, quality of papillar muscles) with patients who remained stable or improved their cardiac function.
Table 3
| Outcome | LVNC (n=20) | Hypertrabeculations (n=14) | P |
|---|---|---|---|
| Cardiac function worsening | 6 | 1 | 0.221 |
| Cardiac transplantation | 1 | 0 | |
| Cardiac function stability | 9 | 11 | 0.172 |
| Cardiac function improvement | 4 | 2 | 0.653 |
| MACE (cardiac, stroke) | 0 | 0 | >0.99 |
LVNC, left ventricular noncompaction; MACE, major acute cardiovascular event.
Discussion
Cardiac MRI is currently used to confirm the diagnosis of LVNC suggested by echocardiography, both techniques displaying a ratio NC/C of at least 2.3 (10). To our knowledge, no study until now has described such a selective distinct method of endocardial borders delineation for measurements of LV volumes, mass and LVEF to help differentiate LVNC from hypertrabeculations. Until the present study, it is likely that LVEF was only calculated including trabeculations within the LV. In one previous study not dedicated to LVNC (11), the comparison of LV function including or excluding papillary muscles and trabeculations showed LVEF variations of as much 3% and of LV mass of 16.6%
The assessment of LV volumes has been previously described in LVNC patients by Jacquier et al. (12), but this study did not provide separate functional data considering compact and global mass. Grothoff et al. (13) reported quite different results, likely as a result of blood pool exclusion. Although LVNC patients had a significant amount of trabeculations particularly located at the apex, the apical and the middle LV segments in two other studies, the presence and extent of trabeculations did not correlate with EF (14,15). In this work we made the assumption that it is possible to indirectly assess the trabeculated fibers behavior by comparing a concurrent LV volume, mass and LVEF with and without trabeculation inclusion between LVNC patients and individuals displaying myocardial hypertrabeculations. These findings warrant the idea that myocardial trabeculae can be indirectly characterized via their compressibility patterns. In this context, the trabeculations should not shorten in LVNC whereas they have a normal contraction in case of simple hypertrabeculations.
Previous studies including LVEF in their data reported conflicting results. For Choi et al. (16), no correlation was observed between trabeculated LV volume and LVEF in LVNC and Control groups. Similar results were provided by Nucifora et al. (17) who concluded that the presence and extent of trabeculations did not correlate with LVEF, whereas Cheng et al. (18) found that the number of trabeculated segments and NC/C ratio had negative correlation with LVEF. None of these studies however included such functional data as in the present study to help discriminate LVNC from hypertrabeculations. Amzulescu et al. reported that the degree of trabeculations in dilated cardiomyopathies was not a prognostic factor, whereas LVEF was a specific independent predictor (19). It is uncertain that trabeculations were included in LV volumes.
Kawel et al. (5), in a large population from the MESA study, found that a NC/C ratio in at least one myocardial segment in diastole was frequent in their study participants, thus suggesting that the current LVNC cardiac MR criteria should be re-actualized.
Along with LVEF changes observed in the present study, another unexpected finding is that in LVNC patients, the mean end-systolic and end-diastolic mass was significantly different when including the trabeculations. This difference does not seem to be the result of inadequate tracings since interobserver agreement was good. Moreover, compact myocardium displayed uniform mass measurements whatever the cardiac phase, as it is reported in the literature (20,21). Conversely, LVEF measurements including trabeculations must not be taken into account for the assessment of LV function as they do not reflect the true cardiac output. Probably the spongy myocardium acts as a blood reservoir similar to the LV chamber itself.
Eccentricity of the left ventricle was found to be a relatively robust landmark of complex infrastructural and functional changes (3,22). However, it did not influence compact and global measurements. Among the other LVNC features, the absence or hypoplasia of papillar muscles has not yet been described to our knowledge.
Finally, some physiological features in LVNC should constitute ominous signs that could differentiate these two entities and select patients that require a strict surveillance or specific therapeutic measures.
This study has several limitations. A positive genotype was present in only 6 LVNC patients; it is acknowledged that genetic penetrance for this cardiomyopathy is not high. Criteria supporting LVNC as a morphological trait shared by different cardiomyopathies may depend on consensus guidelines from the multiple professional organizations (23). Since morphological imaging takes an important part in the diagnosis along with the increased use of genetics, functional data deserve to be included. The participation of LV geometry in LVEF needs to be elucidated, as well as the role of including blood pool. The accuracy of contouring trabeculations and papillary muscles is very subjective, and must take into account than these papillary muscles may be atrophic or missing.
The level of significance of the differences observed is somewhat low and a more important cohort of LVNC patients and controls would be welcome. A direct comparison with echocardiography and/or strain studies is missing. Moreover, since this disease is quite infrequent, the confirmation of the data exhibited by the present study needs further multicentric cooperation projects. Finally, the proposal of including distinct LVEF assessment to diagnostic criteria of LVNC has some caveats, since behavior of hypertrabeculated fibers can be easily and rapidly assessed visually.
Data upon the type of treatment administered in both group of patients are lacking, hence we cannot explain the clinical improvement of some of them at follow up.
Conclusions
Depending on trabeculation inclusion or exclusion, measurements of LV mass and LVEF were significantly different between LVNC patients and hypertrabeculated controls. LV ejection fraction was increased when trabeculations were included in patients with LVNC and conversely in hypertrabeculated normal variants. This data warrants the idea that myocardial trabeculae can be indirectly characterized via their compressibility patterns. Distinctive LVEF might be used as an adjunctive clinical tool to help confirm the diagnosis of LVNC, along with the absence of contraction and/or shortening of trabeculae.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1274/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1274/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Bichat University Hospital. Informed consent was waived with the approval of the institutional review board due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license).
References
- Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet 2015;386:813-25. [Crossref] [PubMed]
- Zuccarino F, Vollmer I, Sanchez G, Navallas M, Pugliese F, Gayete A. Left ventricular noncompaction: imaging findings and diagnostic criteria. AJR Am J Roentgenol 2015;204:W519. [Crossref] [PubMed]
- Boban M, Pesa V, Gabric ID, Manola S, Persic V, Antic-Kauzlaric H, Zulj M, Vcev A. Auxiliary diagnostic potential of ventricle geometry and late gadolinium enhancement in left ventricular non-compaction; non-randomized case control study. BMC Cardiovasc Disord 2017;17:286. [Crossref] [PubMed]
- Dodd JD, Holmvang G, Hoffmann U, Ferencik M, Abbara S, Brady TJ, Cury RC. Quantification of left ventricular noncompaction and trabecular delayed hyperenhancement with cardiac MRI: correlation with clinical severity. AJR Am J Roentgenol 2007;189:974-80. [Crossref] [PubMed]
- Kawel N, Nacif M, Arai AE, Gomes AS, Hundley WG, Johnson WC, Prince MR, Stacey RB, Lima JA, Bluemke DA. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2012;5:357-66. [Crossref] [PubMed]
- Masso AH, Uribe C, Willerson JT, Cheong BY, Davis BR. Left Ventricular Noncompaction Detected by Cardiac Magnetic Resonance Screening: A Reexamination of Diagnostic Criteria. Tex Heart Inst J 2020;47:183-93. [Crossref] [PubMed]
- Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, Hamada H, Hirose O, Isobe T, Yamada K, Kurotobi S, Mito H, Miyake T, Murakami Y, Nishi T, Shinohara M, Seguchi M, Tashiro S, Tomimatsu H. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 1999;34:233-40. [Crossref] [PubMed]
- Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update : Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson 2020;22:19. [Crossref] [PubMed]
- Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15:155-63. [Crossref] [PubMed]
- Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46:101-5. [Crossref] [PubMed]
- Weinsaft JW, Cham MD, Janik M, Min JK, Henschke CI, Yankelevitz DF, Devereux RB. Left ventricular papillary muscles and trabeculae are significant determinants of cardiac MRI volumetric measurements: effects on clinical standards in patients with advanced systolic dysfunction. Int J Cardiol 2008;126:359-65. [Crossref] [PubMed]
- Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY, Vidal V, Bartoli JM, Habib G, Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J 2010;31:1098-104. [Crossref] [PubMed]
- Grothoff M, Pachowsky M, Hoffmann J, Posch M, Klaassen S, Lehmkuhl L, Gutberlet M. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol 2012;22:2699-709. [Crossref] [PubMed]
- Charalampopoulos G, Keramida K, Apostolopoulou SC, Mademli M, Nihoyannopoulos P, Alexopoulou E, Kelekis NL. Prevalence and quantification of left ventricular trabeculation detected by cardiac magnetic resonance in non-ischaemic primary or secondary cardiomyopathies. Hell J Radiol 2018;3:12-20.
- Yildirim G, Dursun M, Arslan R. Effect of trabeculated myocardial mass on left ventricle global and regional functions in noncompaction cardiomyopathy. World J Cardiol 2021;13:211-22. [Crossref] [PubMed]
- Choi Y, Kim SM, Lee SC, Chang SA, Jang SY, Choe YH. Quantification of left ventricular trabeculae using cardiovascular magnetic resonance for the diagnosis of left ventricular non-compaction: evaluation of trabecular volume and refined semi-quantitative criteria. J Cardiovasc Magn Reson 2016;18:24. [Crossref] [PubMed]
- Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail 2011;13:170-6. [Crossref] [PubMed]
- Cheng H, Zhao S, Jiang S, Lu M, Yan C, Ling J, Zhang Y, Liu Q, Ma N, Yin G, Wan J, Yang Y, Li L, Jerecic R, He Z. Comparison of cardiac magnetic resonance imaging features of isolated left ventricular non-compaction in adults versus dilated cardiomyopathy in adults. Clin Radiol 2011;66:853-60. [Crossref] [PubMed]
- Amzulescu MS, Rousseau MF, Ahn SA, Boileau L, de Meester de Ravenstein C, Vancraeynest D, Pasquet A, Vanoverschelde JL, Pouleur AC, Gerber BL. Prognostic Impact of Hypertrabeculation and Noncompaction Phenotype in Dilated Cardiomyopathy: A CMR Study. JACC Cardiovasc Imaging 2015;8:934-46. [Crossref] [PubMed]
- Bricq S, Frandon J, Bernard M, Guye M, Finas M, Marcadet L, Miquerol L, Kober F, Habib G, Fagret D, Jacquier A, Lalande A. Semiautomatic detection of myocardial contours in order to investigate normal values of the left ventricular trabeculated mass using MRI. J Magn Reson Imaging 2016;43:1398-406. [Crossref] [PubMed]
- Captur G, Radenkovic D, Li C, Liu Y, Aung N, Zemrak F, Tobon-Gomez C, Gao X, Elliott PM, Petersen SE, Bluemke DA, Friedrich MG, Moon JC. Community delivery of semiautomated fractal analysis tool in cardiac mr for trabecular phenotyping. J Magn Reson Imaging 2017;46:1082-8. [Crossref] [PubMed]
- Laissy JP, Andrianarimanitra H, Haioun K, Wlachovska B, Ben Driss A. Feasibility of Virtual 3D Cardiac CT Angioscopy to Help Discriminate Left Ventricular Non-Compaction from Hypertrabeculations. A Preliminary Case Control Report. J Clin Images 2023;6:1147.
- Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol 2014;64:1840-50. [Crossref] [PubMed]


