Predicting asymptomatic coronary artery disease in first-ever acute ischemic stroke patients: a cross-sectional study
Introduction
Stroke is the second leading cause of mortality globally and one of the most prevalent neurological disorders (1,2). Approximately 80–90% of all strokes are ischemic strokes (3). Coronary artery disease (CAD) and ischemic stroke share common risk factors and often coexist. Moreover, more than 60% of cases of ischemic stroke have asymptomatic CAD, and 16–35% of cases of ischemic stroke have severe CAD (coronary arterial stenosis of >50%) (4). Furthermore, asymptomatic CAD in acute ischemic stroke (AIS) is predictive of stroke recurrence and poor survival besides cardiovascular events. When compared to individuals without asymptomatic CAD, major vascular events are more likely to occur in stroke patients with asymptomatic CAD, and the risk of such events risk rises as the extent of CAD increases (5). Therefore, screening and analyzing patients with AIS with CAD could help to develop clinical therapeutic strategies, predict long-term prognosis, and lower the recurrence of CAD and AIS.
Atherosclerosis is a chronic systemic disease known to be the leading cause of AIS and asymptomatic CAD. The atherosclerotic disease present in one area can predict ischemic episodes in other locations (6). In this context, carotid arteries have been used to assess the risk of CAD (7). The presence and severity of CAD are linked to carotid plaques (8,9). However, for asymptomatic CAD, some studies have failed to confirm the predictive value of carotid plaques and intima-media thickness (10,11). Recently, considerable attention has been paid to the association between aortic atherosclerosis and the incidence or recurrence of ischemic stroke. Aortic atheroma, an atherosclerotic plaque in the descending thoracic aorta, aortic arch, or ascending aorta, affects 14–42% of individuals with ischemic cerebrovascular disease (12). Besides, aortic arch plaque (AAP) is one of the first signs of systemic atherosclerosis. The presence of AAP is probably a promising early indicator of asymptomatic CAD in AIS patients, allowing for more targeted and integrated treatment. However, just a few preliminary investigations explored the association between AAP and asymptomatic CAD in AIS patients and came to conflicting conclusions (13-15), and so far, no study has shown the relationship between AAP and asymptomatic CAD in first-ever AIS patients.
Combined coronary and cervicocephalic computed tomography angiography (CTA) with a single bolus of contrast agent is now possible due to improvements in noninvasive imaging techniques (16,17). In this study, we hypothesized that the presence and severity of AAP can serve as an early indicator of asymptomatic CAD in patients with first-ever AIS. We aimed to assess the association between AAP and asymptomatic CAD, as well as compare the predictive power of AAP with traditional cervical and intracranial atherosclerotic characteristics using combined coronary and cervicocephalic CTA. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1405/rc).
Methods
Participants
Consecutive patients admitted to Xuanwu Hospital, Capital Medical University with a first-ever AIS within 2 weeks after the symptom onset were enrolled in this single-center cross-sectional study from January 2019 to December 2021. Patients who underwent both routine brain magnetic resonance imaging (MRI) and combined coronary and cervicocephalic CTA were included for analysis. According to our definition, ischemic stroke occurs when localized neurological impairments appear suddenly and remain for longer than 24 hours with corresponding medical imaging evidence (positive diffusion-weighted imaging findings) on MRI. Patients with first-ever AIS caused by large arterial atherosclerosis were included in this study based on Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (18). The exclusion criteria were as follows: (I) a history of recognized cardiovascular disease [i.e., stroke, transient ischemic attack (TIA) episode, myocardial infarction, stable or unstable angina pectoris, and revascularization procedure for cardiovascular disease]; (II) suspicion of nonatherosclerotic arterial stenosis (e.g., vasculitis or dissection) and atrial fibrillation related to cardioembolism; (III) complete carotid occlusions as the complete occlusion state may go beyond the scope of plaque assessment and affect the evaluation of plaques; and (IV) those with inadequate organ functioning or other MRI and CTA exclusion criteria. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (No. 2018065) and informed consent was provided by all individual participants.
Demographic features and risk factors
Baseline demographics, clinical characteristics, tests to obtain vascular risk factors, face-to-face interviews, and laboratory blood examination were collected and performed. The National Institute of Health Stroke Scale (NIHSS) score at admission was recorded. The vascular risk factors analyzed in this study were smoking history (current smoker or having a regular smoking habit in the past), hypertension, dyslipidemia, diabetes mellitus, and hyperhomocysteinemia, which were defined based on interview or laboratory standard diagnosis. Any first-degree relative’s history of an ischemic cardiovascular event (myocardial infarction, stroke, or TIA) was noted.
CT scan protocols and data reconstruction
Using the dual-source 192-slice CT scanner (Somatom Force, Siemens Healthcare, Erlangen, Germany), all combined coronary and cervicocephalic CTAs were conducted out as previously reported (19). The CTA scans were conducted from the base of the heart to the head cauda-cranially, using an automated kVp and mA (CARE kV and CARE Dose4D, Siemens) with a pitch of 3.2 and a rotation time of 250 ms. Trigger phases for 60% or 30% of the RR interval were selected for heart rates below and above 70 bpm, respectively. Using a power injector and a flow rate of 5 mL/s, a total of 40 mL of contrast material (Ultravist 370 Iodine/mL; Bayer Schering Pharma, Leverkusen, Germany) was intravenously administered via the antecubital vein. This was followed by a 50 mL saline chaser bolus at the same pace. Data acquisition was prompted with a mean delay of 8 seconds after the attenuation threshold was reached during the bolus-tracking technique in the ascending aorta (attenuation threshold, 100 HU). In the case of raw data, an advanced model-based iterative reconstruction using a medium smooth reconstruction kernel (Bv36) was applied. The reconstruction slice thickness and interval were both 0.6 mm. Each CTA image dataset was transferred to the Syngo.via workstation (MMWP, Syngo.via, Siemens) for artery stenosis evaluation and subsequent plaque characteristic analysis.
Plaque analysis for the carotid bifurcation and aortic arch
The carotid arteries comprised the left and right carotid arteries within 3 cm proximal and distal of the bifurcation. The aortic arch was assessed from the aortic root to the left subclavian artery’s distal end. Based on the most predominant type of plaque, calcified, noncalcified, and mixed AAPs and carotid bifurcation plaques were distinguished (20). AAPs and carotid bifurcation plaques were evaluated by measuring them in consecutive, evenly spaced cross-sections with a consistent interval of 2 mm between each perpendicular slice. The thickness of the AAPs and carotid bifurcation plaques was calculated as the distance between the maximum plaque’s highest point and the wall of the aortic or carotid artery’s outer membrane (21). Moreover, AAPs were assessed based on their presence and extent, which was graded as either absent, mild (few, minor plaques), or severe (frequent, large plaques) (22). The surface morphology of the AAPs was classified into 3 types (smooth, irregular, or ulcerative AAP). Smooth AAP indicated a plaque with regular luminal shape; outpouching of the contrast material into or adjacent to the plaque at least 1 mm in the carotid artery and 2 mm in the aortic arch was considered ulcerative AAP (21); irregular AAP was defined as that a plaque surface shape revealed irregularities without any indications of ulceration (23).
Stenosis assessment
Given that none of the chosen patients with AIS had a history of CAD, the presence of stenosis of more than 50% in at least 1 coronary artery segment indicated the presence of asymptomatic CAD. Only coronary artery segments with a diameter of more than 1.5 mm were examined (24). The presence of carotid-cerebral atherosclerosis was determined in arteries or segments that were divided into 2 categories, including extracranial arteries and intracranial arteries. Extracranial arteries include the bilateral proximal subclavian arteries, common carotid arteries, extracranial segments of the internal carotid arteries, and extracranial segments of the vertebral arteries. Intracranial arteries include the bilateral intracranial segments of the internal carotid arteries, intracranial segments of the vertebral arteries, basilar artery, as well as the bilateral anterior cerebral arteries, middle cerebral arteries, and posterior cerebral arteries. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) method was used to determine the degree of stenosis in each artery and segment (25). Significant atherosclerotic stenosis of carotid-cerebral arteries was defined as stenosis of at least 50%. All CTA data were independently assessed and agreed upon by 2 board-certified radiologists with more than 5 years of experience.
Grouping of study participants
According to the presence or absence of asymptomatic CAD on combined coronary and cervicocephalic CTA, the examined patients with first-ever AIS were split into the asymptomatic CAD and non-CAD groups.
Statistical analysis
Statistical analyses were performed using the software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc 15.0 (MedCalc, Ostend, Belgium). The normality of the numerical variables was determined by the histograms and Kolmogorov-Smirnov tests. The numerical variables with normal distribution were presented as means ± standard deviations (SD) and tested by Student’s t-test, whereas the non-normally distributed variables were presented as medians together with interquartile ranges (IQRs) and tested by Mann-Whitney U test. Categorical variables were shown as counts together with percentages, and the chi-square tests or Fisher’s exact tests were used for statistical tests as appropriate. The general characteristics and the atherosclerosis characteristics between the asymptomatic CAD and non-CAD groups were compared in the univariate analysis. Statistical significance was defined as a 2-sided P value less than 0.05. The multivariate logistic regression analyses were performed to examine the independent associations between atherosclerosis features and the presence of asymptomatic CAD in patients with AIS by adjusting covariates. The covariates were selected according to the univariate analysis results (P<0.05) of general characteristics. The backward stepwise logistic regression analysis (with the likelihood ratio method as the variable selection strategy) was used to examine the predictive effect of different atherosclerosis characteristics for asymptomatic CAD in AIS after adjusting for covariates. Both the entry and exit significance levels of the stepwise regression model were set at 0.1. The predictive performance of logistic regression models was evaluated by the area under the receiver operating characteristic curves (AUC). The comparison of AUC values of different models was done by the Delong test. Odds ratios (OR) for the risk of the presence of asymptomatic CAD in AIS and AUC values were calculated with 95% confidence intervals (CI).
Results
Participant characteristics
A total of 182 patients with AIS were enrolled (Figure 1). The patients incorporated were primarily males (86.8 %), with mean age of 60.0 years. Combined coronary and cervicocephalic CTA showed asymptomatic CAD in 84 of the 182 (46.2%) patients with AIS. Table 1 lists the general characteristics of the 182 patients. Smoking (72.6% vs. 54.1%; P=0.010) and diabetes mellitus (54.8% vs. 39.8%; P=0.044) significantly differed between patients with asymptomatic CAD and those without.
Table 1
| Characteristics | All patients | Asymptomatic CAD group (n=84) |
Non-CAD group (n=98) |
Statistics | P value |
|---|---|---|---|---|---|
| Age (years) | 60.0±9.6 | 61.1±8.4 | 59.0±10.4 | t (180) =1.482, | 0.140 |
| Male | 158 (86.8) | 77 (91.7) | 81 (82.7) | χ2(1) =3.210 | 0.073 |
| BMI (kg/m2) | 24.9±3.1 | 24.7±3.1 | 25.2±3.1 | t (180) =−1.076 | 0.284 |
| NIHSS on admission | 3 [1, 5] | 3 [1, 5] | 3 [1, 5] | Z=0.320 | 0.749 |
| History of HTN | 135 (74.2) | 65 (77.4) | 70 (71.4) | χ2(1) =0.837 | 0.360 |
| History of HLP | 102 (56.0) | 45 (53.6) | 57 (58.2) | χ2(1) =0.387 | 0.534 |
| History of DM | 85 (46.7) | 46 (54.8) | 39 (39.8) | χ2(1) =4.070 | 0.044 |
| Smoking | 114 (62.6) | 61 (72.6) | 53 (54.1) | χ2(1) =6.641 | 0.010 |
| Hyperhomocysteinemia | 31 (17.0) | 16 (19.0) | 15 (15.3) | χ2(1) = 0.448 | 0.503 |
| Family history of CAD | 16 (8.8) | 8 (9.5) | 8 (8.2) | χ2(1) =0.104 | 0.747 |
| Family history of stroke | 5 (2.7) | 5 (5.9) | 0 (0.0) | χ2(1) =0.115 | 0.750 |
The data are shown as means ± standard deviations or n (%) or median [Q1, Q3] as appropriate. AIS, acute ischemic stroke; CAD, coronary artery disease; BMI, body mass index; NIHSS, national institute of health stroke scale; HTN, hypertension; HLP, hyperlipidemia; DM, diabetes mellitus.
Comparison of the atherosclerosis characteristics between the asymptomatic CAD and non-CAD groups
Among 182 patients, 91 (50.0%) had cervical atherosclerotic stenosis of ≥50% and 145 patients (79.7%) had intracranial atherosclerotic stenosis ≥50%. The most common type of carotid bifurcation plaque was mixed plaque (46.2%), and the largest carotid bifurcation plaque mean thickness was 2.64 mm. The AIS patients with asymptomatic CAD were more likely to have intracranial atherosclerotic stenosis (86.9% vs. 73.5%; P=0.025) than those without asymptomatic CAD (Table 2 and Figure 2). However, there was no significant difference between the 2 groups in the presence of cervical atherosclerotic stenosis of more than 50% (57.1% vs. 43.9%; P=0.074). No statistical differences in the type of the largest carotid bifurcation plaque and the thickness of the largest carotid bifurcation plaque were found between the 2 groups (P=0.249 and P=0.052; Table 2 and Figure 2).
Table 2
| Characteristics | Asymptomatic CAD group (N=84) | Non-CAD group (N=98) | Statistics | P value |
|---|---|---|---|---|
| Cervical atherosclerotic stenosis ≥50% | 48 (57.1) | 43 (43.9) | χ2(1) =3.18 | 0.074 |
| Intracranial atherosclerotic stenosis ≥50% | 73 (86.9) | 72 (73.5) | χ2(1) =5.04 | 0.025 |
| Max CBP thickness, mm | 2.95 [1.37, 4.32] | 2.45 [0.00, 3.75] | Z=−1.94 | 0.052 |
| Max CBP type | χ2(3) =4.12 | 0.249 | ||
| None | 15 (17.9) | 26 (26.5) | ||
| Hard | 19 (22.6) | 20 (20.4) | ||
| Soft | 6 (7.1) | 12 (12.2) | ||
| Mixed | 44 (52.4) | 40 (40.8) | ||
| AAP extent | χ2(3) =16.29 | <0.001 | ||
| None | 16 (19.0) | 26 (26.5) | ||
| Mild | 40 (47.6) | 63 (64.3) | ||
| Severe | 28 (33.3) | 9 (9.2) | ||
| AAP type | χ2(3) =8.60 | 0.035 | ||
| None | 16 (19.0) | 27 (27.6) | ||
| Hard | 30 (35.7) | 45 (45.9) | ||
| Soft | 9 (10.7) | 10 (10.2) | ||
| Mixed | 29 (34.5) | 16 (16.3) | ||
| Complex AAP | – | <0.001 | ||
| None | 53 (63.1) | 84 (85.7) | ||
| Smooth | 2 (2.4) | 6 (6.1) | ||
| Irregular | 19 (22.6) | 7 (7.1) | ||
| Ulcer | 10 (11.9) | 1 (1.0) | ||
| AAP thickness, mm | 2.75 [1.20, 5.10] | 1.90 [0.00, 2.90] | Z=−2.95 | 0.003 |
The data are shown as medians [IQRs] or n (%) as appropriate. Fisher’s exact test was used for comparing complex AAP between patients with AIS with and without asymptomatic CAD. AIS, acute ischemic stroke; CAD, coronary artery disease; IQR, interquartile range; CBP, carotid bifurcation plaque; AAP, aortic arch plaque.

The AAPs in the asymptomatic CAD group were thicker (2.75 vs. 1.90 mm; P=0.003) and had more frequent severe AAP (33.3% vs. 9.2%; P<0.001), mixed AAP type (34.5% vs. 16.3%, P=0.035), and ulcerative AAP (11.9% vs. 1.0%, P<0.001) than the non-CAD group (Table 2 and Figures 2-4).
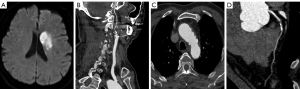
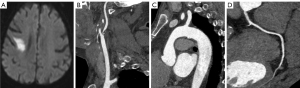
In 11 patients with AIS who had ulcerative AAP, the likelihood of developing asymptomatic CAD was 90.9% (10/11). In 137 patients with AIS without complex AAP, the possibility of developing asymptomatic CAD was only 38.7% (53/137).
Multivariate logistic regression on risk factors
The comparison of the atherosclerosis characteristics between the groups with and without asymptomatic CAD were performed using the multiple logistic regression model with the adjustment covariates of smoking and diabetes. The adjusted P value and adjusted odds ratio (aOR) with 95% CI of each atherosclerosis characteristic are shown in Table 3. After adjusting for covariates, increased AAP thickness per millimeter (aOR: 1.26; 95% CI: 1.08–1.47), severe AAP (aOR: 4.24; 95% CI: 1.59–12.03), the presence of mixed AAP (aOR: 2.65; 95% CI: 1.09–6.62), and the presence of ulcerative AAP (aOR: 11.76; 95% CI: 2.05–222.84) remained associated with concomitant asymptomatic CAD (Table 3).
Table 3
| Characteristics | Coefficient | SD | Adjusted P value | aOR [95% CI] | AUC [95% CI] |
|---|---|---|---|---|---|
| Cervical atherosclerotic stenosis ≥50% | 0.54 | 0.31 | 0.080 | 1.72 [0.94, 3.19] | 0.66 [0.58, 0.74] |
| Intracranial atherosclerotic stenosis ≥50% | 0.88 | 0.42 | 0.035 | 2.41 [1.09, 5.61] | 0.67 [0.60, 0.75] |
| Max CBP thickness, mm | 0.14 | 0.08 | 0.079 | 1.15 [0.99, 1.35] | 0.68 [0.60, 0.76] |
| Max CBP type | 0.67 [0.59, 0.74] | ||||
| None | Reference | ||||
| Hard | 0.29 | 0.48 | 0.541 | 1.34 [0.53, 3.43] | |
| Soft | −0.22 | 0.62 | 0.726 | 0.81 [0.23, 2.65] | |
| Mixed | 0.54 | 0.40 | 0.180 | 1.72 [0.78, 3.86] | |
| AAP extent | 0.69 [0.62, 0.77] | ||||
| None | Reference | ||||
| Mild | −0.03 | 0.39 | 0.933 | 0.97 [0.46, 2.09] | |
| Severe | 1.44 | 0.51 | 0.005 | 4.24 [1.59, 12.03] | |
| AAP type | 0.67 [0.59, 0.75] | ||||
| None | Reference | ||||
| Hard | 0.10 | 0.40 | 0.811 | 1.10 [0.50, 2.46] | |
| Soft | 0.30 | 0.57 | 0.595 | 1.36 [0.44, 4.20] | |
| Mixed | 0.97 | 0.46 | 0.033 | 2.65 [1.09, 6.62] | |
| Complex AAP | 0.71 [0.64, 0.79] | ||||
| None | Reference | ||||
| Smooth | −0.66 | 0.86 | 0.445 | 0.52 [0.07, 2.47] | |
| Irregular | 1.43 | 0.49 | 0.004 | 4.20 [1.66, 11.71] | |
| Ulcer | 2.46 | 1.08 | 0.023 | 11.76 [2.05, 222.84] | |
| AAP thickness, mm | 0.23 | 0.08 | 0.003 | 1.26 [1.08, 1.47] | 0.69 [0.61, 0.77] |
AIS, acute ischemic stroke; CAD, coronary artery disease; SD, standard deviation of the logistic regression coefficient; aOR, adjusted odds ratio; OR, odds ratio for the risk of the presence of asymptomatic CAD in AIS; 95% CI, 95 percent of confidence interval; AUC, area under the curve; CBP, carotid bifurcation plaque; AAP, aortic arch plaque.
Predictive power of the atherosclerosis characteristics for asymptomatic CAD in AIS
The predictive power of each atherosclerosis characteristic is shown using AUC of the predicted probabilities of different logistic regression models in Table 3. The model with complex AAP showed the highest AUC of 0.71 (95% CI: 0.64–0.79). The backward stepwise logistic model was built by atherosclerosis characteristics after adjusting for smoking and diabetes. The final model is shown in Table 4; complex AAP was retained.
Table 4
| Variables | Coefficient | SD | OR [95% CI] | P value |
|---|---|---|---|---|
| Intercept | −1.22 | 0.34 | – | – |
| Smoking | 0.73 | 0.34 | 2.08 [1.07, 4.14] | 0.033 |
| Diabetes mellitus | 0.68 | 0.33 | 1.97 [1.03, 3.83] | 0.041 |
| Complex AAP | ||||
| None | Reference | |||
| Smooth | −0.66 | 0.86 | 0.52 [0.07, 2.47] | 0.445 |
| Irregular | 1.43 | 0.49 | 4.20 [1.66, 11.71] | 0.004 |
| Ulcer | 2.46 | 1.08 | 11.76 [2.05, 222.84] | 0.023 |
The final predictive model: logit (the probability of the presence of asymptomatic CAD) = −1.22 + 0.73 × smoking history + 0.68 × diabetes mellitus history – 0.66 × smooth AAP + 1.43 × irregular AAP + 2.46 × ulcerative AAP. CAD, coronary artery disease; AIS, acute ischemic stroke; SD, standard deviation of the logistic regression coefficient; OR, odds ratio for the risk of the presence of asymptomatic CAD in AIS; 95% CI, 95% confidence interval; AAP, aortic arch plaque.
To determine whether AAP could provide more predictive power than cervicocephalic atherosclerotic stenosis ≥50% and carotid bifurcation plaque in predicting asymptomatic CAD, first, we built a basic logistic regression model only including smoking and diabetes to predict the presence of asymptomatic CAD in AIS (AUC of the basic model: 0.64; 95% CI: 0.56–0.71; P=0.008). Then, we separately added the 3 types of predictors into the basic model. The results showed that entering the AAP characteristics significantly increased the basic model’s predictive power (changes in the AUC: 0.07 for complex AAP, P=0.008) (Figure 5 and Table 5). Meanwhile, adding cervicocephalic atherosclerotic stenosis ≥50% and carotid bifurcation plaque did not improve the predictive power of the basic model significantly (changes in the AUC: 0.04 for cervicocephalic atherosclerotic stenosis ≥50%, P=0.122; 0.04 for carotid bifurcation plaque, P=0.097).
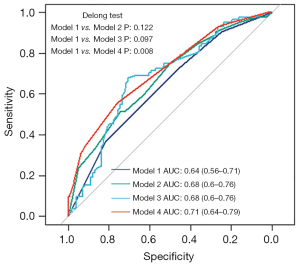
Table 5
| Predictors | Model 1 | Model 2 | Model 3 | Model 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR [95% CI] | P value | OR [95% CI] | P value | OR [95% CI] | P value | OR [95% CI] | P value | ||||
| Smoking | 2.49 [1.32, 4.79] | 0.005 | 2.65 [1.39, 5.18] | 0.004 | 2.41 [1.27, 4.70] | 0.008 | 2.08 [1.07, 4.14] | 0.033 | |||
| Diabetes mellitus | 2.05 [1.12, 3.83] | 0.021 | 1.86 [1.00, 3.51] | 0.053 | 2.03 [1.08, 3.88] | 0.029 | 1.97 [1.03, 3.83] | 0.041 | |||
| Cervical atherosclerotic stenosis ≥50% | 1.51 [0.81,2.84] | 0.198 | |||||||||
| Intracranial atherosclerotic stenosis ≥50% | 2.12 [0.94,5.04] | 0.077 | |||||||||
| Max CBP type | |||||||||||
| None | Reference | ||||||||||
| Hard | 0.90 [0.30, 2.67] | 0.849 | |||||||||
| Soft | 0.41 [0.08, 1.86] | 0.256 | |||||||||
| Mixed | 0.78 [0.19, 3.01] | 0.721 | |||||||||
| Max CBP thickness, mm | 1.22 [0.93, 1.63] | 0.164 | |||||||||
| Complex AAP | |||||||||||
| None | Reference | ||||||||||
| Smooth | 0.52 [0.07, 2.47] | 0.445 | |||||||||
| Irregular | 4.20 [1.66, 11.71] | 0.004 | |||||||||
| Ulcer | 11.76 [2.05, 222.84] | 0.023 | |||||||||
Model 1 only includes vascular risk factors (smoking and diabetes) in the basic logistic model; model 2 adds features of cervical atherosclerotic stenosis ≥50% and intracranial atherosclerotic stenosis ≥50% in addition to smoking and diabetes; model 3 adds max CBP type and max carotid bifurcation plaque thickness in addition to smoking and diabetes; model 4 adds complex AAP in addition to smoking and diabetes. CAD, coronary artery disease; AIS, acute ischemic stroke; OR, odds ratio for the risk of the presence of asymptomatic CAD in AIS; 95% CI, 95 percent of confidence interval; CBP, carotid bifurcation plaque; AAP, aortic arch plaque.
Discussion
A combined CTA in this investigation was implemented to simultaneously evaluate atherosclerosis of the cervicocephalic arteries, aortic arch, and coronary artery in individuals with AIS who had no prior history of CAD. Complex AAP, especially ulcerative AAP, was correlated to the existence of asymptomatic CAD. The analytic model with AAP ulceration with conventional vascular risk factors improved the predictive power for asymptomatic CAD.
The prevalence of asymptomatic CAD in patients with first-ever AIS included in this study was 46.2%, which is similar to that reported in previous studies (18–48.3%) (24,26,27). Although ample evidence indicates the risk of CAD in stroke patients, it remains unclear to what extent this risk can be predicted by stenosis or the presence of arterial plaques in extracardiac locations (e.g., cervicocephalic arteries or aortic arch). Therefore, this study aimed to investigate the potential value of the atherosclerotic features of cervicocephalic CTA in predicting asymptomatic CAD in patients with first-ever AIS. The goal was to determine whether further coronary CTA examinations were necessary for these patients.
Atherosclerosis is a systemic and progressive disease, with vascular stenosis being an important manifestation during its development. The American Heart Association (AHA) and American Stroke Association (ASA) recommend noninvasive CAD testing for individuals with carotid atherosclerotic stenosis and strong vascular risk factors (28). According to the findings of this study, patients with first-ever AIS who also had asymptomatic CAD were more likely to have intracranial atherosclerotic stenosis ≥50% than those without asymptomatic CAD; however, there was no significant difference in cervical atherosclerotic stenosis between the 2 groups. Previous research has suggested that intracranial atherosclerotic stenosis ≥50% may be able to assist in identifying cerebral infarct patients who are at highest risk of developing asymptomatic CAD (29). However, the results from receiver operating characteristic (ROC) curve analysis in this study showed that cervical and intracranial atherosclerotic stenosis ≥50% did not improve the predictive power of vascular risk factor exposure for asymptomatic CAD.
Although hypoperfusion and the consequent sluggish flow from cervicocephalic atherosclerotic undoubtedly contribute to a portion of strokes occurring in cervicocephalic stenosis, plaque instability leading to distal embolus may be a substantially more significant etiological component for stroke in cervicocephalic atherosclerotic disease. It may be necessary to consider plaque characteristics beyond only the degree of stenosis in order to detect patients with high risk of developing asymptomatic CAD. According to the CTA results, carotid bifurcation plaque was divided into 3 categories: calcified plaque made up mostly of calcified tissue (>130 HU), soft plaque made up primarily of noncalcified tissue, and mixed plaque made up of both calcified and noncalcified tissues (20). This study found no significant difference in the composition of the max carotid bifurcation plaque between patients with AIS with and without asymptomatic CAD. According to Gupta et al., there is the direct correlation between symptomatic carotid bifurcation plaque and rising soft plaque thickness; moreover, they reported that soft carotid bifurcation plaque thickness predicts ischemic stroke risk (30).
Rarely was the significance of AAP for predicting asymptomatic CAD in individuals with AIS assessed. Compared to earlier research (13,28), we analyzed the characteristics of AAP more comprehensively and investigated the predictive value of AAP features for asymptomatic CAD in AIS patients with the help of statistical modeling. In accordance with our findings, Cho et al. (13) proposed that the existence of complicated AAP on CTA (thickness of 4 mm or protruded or ulcerated) was a 50% predictor of coronary stenosis in individuals with AIS who had no history of CAD (aOR: 5.71; 95% CI: 1.94–16.87). They focused on complicated AAP, which could be a cause of AIS on its own, but only partially reflected the AAP features. More AAP characteristics were required to adequately illustrate the link between AAP and asymptomatic CAD. Our study simultaneously evaluated the extent, thickness, plaque type, and complexity characteristics of AAPs. Comparing the AAPs in the asymptomatic CAD group to those in the non-CAD group, we found that the AAPs in the asymptomatic CAD group had thicker thickness (2.75 vs. 1.90 mm; P=0.003), more frequent severe AAP (33.3% vs. 9.2%; P<0.001), more frequent mixed AAP (34.5% vs. 16.3%, P<0.001), and more frequent ulcerative AAP (aOR: 11.76; 95% CI: 2.05–222.84). Although the AAP features described above differed between the 2 groups, the predictive value of them for asymptomatic CAD was still limited. Therefore, additional research was required, such as using high-resolution MRI to determine the relationship between AAP characteristics and asymptomatic CAD. However, this study demonstrated that AAP ulceration was a relatively better indicator of asymptomatic CAD than cervicocephalic atherosclerosis. Plaque ulceration was shown to be one of the characteristics of vulnerable plaque (23), and plaque rupture might be accompanied by thrombus development and embolism of plaque debris or thrombus into the bloodstream to result in ischemic vascular events. Furthermore, considering that various diseases, including cardioembolism, atherosclerosis of the large arteries, small vascular obstruction, and cryptogenic stroke, contribute to the variability of ischemic stroke, may misrepresent the clinical behavior of AAP, our study only included patients with large arterial atherosclerosis according to TOAST classification. Additionally, cervicocephalic artery stenosis, carotid bifurcation plaque, and AAP were simultaneously evaluated in the study and were compared for their ability to predict the development of asymptomatic coronary heart disease. AAP ulceration was shown to be a valuable indicator in asymptomatic CAD prediction, whereas carotid bifurcation plaque characteristics and cervicocephalic artery stenosis showed relatively poor ability to independently predict AIS patients with asymptomatic CAD. The diagnostic performance of the basic clinical risk factor model was improved when AAP ulcers were added, whereas the addition of cervicocephalic artery stenosis and carotid bifurcation plaque features did not improve the diagnostic performance of the basic model. The findings above may indicate that aortic atherosclerosis is more closely connected to coronary atherosclerosis and occurs earlier than extracranial and intracranial atherosclerosis (31). Another possible explanation is that the aorta, extracranial, and intracranial arteries have different anatomical structures, resulting in variations in hemodynamics that alter plaque vulnerability. More extensive research is required to verify the aforementioned theories and delve into their pathophysiological processes.
Atherosclerotic lesions in the thoracic aorta detected by transesophageal echocardiography (TEE) have been shown to be associated with the occurrence and severity of asymptomatic CAD (32,33) and an increased risk of recurrent stroke (34). However, there is limited research specifically focusing on patients with first-ever stroke. Our study used combined CT to evaluate 4 aspects of AAP plaque features, including thickness, extent, composition, and complexity and discovered that AAP ulceration has the best predictive performance for the presence of asymptomatic CAD in AIS patients. This may also indicate that plaque surface morphology, rather than other plaque characteristics, is a better predictor of plaque vulnerability. All in all, we should be aware of the potential coexistence of asymptomatic CAD when we observe AAP ulceration in AIS patients, which is helpful for clinical therapy and risk classification of such individuals. The patient may have asymptomatic CAD and require further coronary examination, as evidenced by the discovery of AAP ulceration on the patient’s cervicocephalic artery CTA. Conversely, asymptomatic CAD is less likely, which means that unnecessary examinations may be avoided, lowering radiation exposure and patient costs.
This study has several limitations. Firstly, affirmative and generalizable conclusions may not be possible given the single-center study’s relatively small sample size of first-ever AIS patients. More studies with larger samples are needed to verify and generalize the conclusions prompted here. Secondly, based on the TOAST classification, there are 5 different forms of AIS. As a result, the performance of the prediction may vary by the etiologies of stroke. Thirdly, although AUC values can partially reflect the predictive value of a diagnostic test and help compare the predictive power of different models, it cannot entirely represent the predictive capacity of models. More indicators are needed to comprehensively evaluate the predictive value from various aspects. Additionally, it is important to acknowledge that the observed wide 95% CI in this study, particularly for AAP ulcerative plaques, is possibly influenced by the relatively small sample size, introducing variability and impacting the precision of the estimates. Moreover, our study did not utilize TEE which is the gold standard for evaluation of AAP; CTA has a limited resolution in AAP imaging. However, CTA is a non-invasive imaging modality that can evaluate both atherosclerotic AAP and provide routine examination of cervical and intracranial arteries in patients with AIS. Lastly, further study requires long-term follow-up examinations to elucidate the prognostic value of AAP ulceration.
Conclusions
In conclusion, the first-ever AIS patients with asymptomatic CAD, are more likely to have thicker, more severe, mixed-type, and ulcerative AAP compared to patients with AIS alone. AAP ulceration is probably a more powerful predictor of the presence of asymptomatic CAD in patients with AIS than cervicocephalic stenosis characteristics. AAP evaluation is crucial for first-ever AIS patients without a history of CAD as it can help to identify asymptomatic CAD early and initiate more integrated therapeutic care.
Acknowledgments
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1405/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1405/coif). All authors report that this research was funded by the Beijing Natural Science Foundation (No. Z190014). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (No. 2018065) and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Katan M, Luft A. Global Burden of Stroke. Semin Neurol 2018;38:208-11. [Crossref] [PubMed]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Baran G, Gultekin TO, Baran O, Deniz C, Katar S, Yildiz GB, Asil T. Association between etiology and lesion site in ischemic brainstem infarcts: a retrospective observational study. Neuropsychiatr Dis Treat 2018;14:757-66. [Crossref] [PubMed]
- Liegey JS, Fawaz S, Ducos C, Pucheu Y, Boulestreau R, Sibon I, Couffinhal T. Predictive utility of stress tests in the detection of asymptomatic coronary artery disease in atherosclerotic stroke patients. J Stroke Cerebrovasc Dis 2023;32:107290. [Crossref] [PubMed]
- Yoo J, Song D, Baek JH, Kim K, Kim J, Song TJ, Lee HS, Choi D, Kim YD, Nam HS, Heo JH. Poor long-term outcomes in stroke patients with asymptomatic coronary artery disease in heart CT. Atherosclerosis 2017;265:7-13. [Crossref] [PubMed]
- Zheng C, Yan S, Fu F, Zhao C, Guo D, Wang Z, Lu J. Cervicocephalic Spotty Calcium for the Prediction of Coronary Atherosclerosis in Patients With Acute Ischemic Stroke. Front Neurol 2021;12:659156. [Crossref] [PubMed]
- Nambi V, Pedroza C, Kao LS. Carotid intima-media thickness and cardiovascular events. Lancet 2012;379:2028-30. [Crossref] [PubMed]
- Selwaness M, Bos D, van den Bouwhuijsen Q, Portegies ML, Ikram MA, Hofman A, Franco OH, van der Lugt A, Wentzel JJ, Vernooij MW. Carotid Atherosclerotic Plaque Characteristics on Magnetic Resonance Imaging Relate With History of Stroke and Coronary Heart Disease. Stroke 2016;47:1542-7. [Crossref] [PubMed]
- Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, Kavousi M, van der Lugt A. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. J Am Coll Cardiol 2021;77:1426-35. [Crossref] [PubMed]
- Polak JF, Backlund JC, Budoff M, Raskin P, Bebu I, Lachin JMDCCT/EDIC Research Group. Coronary Artery Disease Events and Carotid Intima-Media Thickness in Type 1 Diabetes in the DCCT/EDIC Cohort. J Am Heart Assoc 2021;10:e022922. [Crossref] [PubMed]
- Brunner G, Virani SS, Sun W, Liu L, Dodge RC, Nambi V, Coresh J, Mosley TH, Sharrett AR, Boerwinkle E, Ballantyne CM, Wasserman BA. Associations Between Carotid Artery Plaque Burden, Plaque Characteristics, and Cardiovascular Events: The ARIC Carotid Magnetic Resonance Imaging Study. JAMA Cardiol 2021;6:79-86. [Crossref] [PubMed]
- Kong Q, Ma X. Contributing Mechanisms of Aortic Atheroma in Ischemic Cerebrovascular Disease. J Stroke Cerebrovasc Dis 2015;24:2653-9. [Crossref] [PubMed]
- Cho HJ, Lee JH, Kim YJ, Moon Y, Ko SM, Kim HY. Comprehensive evaluation of coronary artery disease and aortic atherosclerosis in acute ischemic stroke patients: usefulness based on Framingham risk score and stroke subtype. Cerebrovasc Dis 2011;31:592-600. [Crossref] [PubMed]
- Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Coronary artery disease and risk of major vascular events after cerebral infarction. Stroke 2013;44:1505-11. [Crossref] [PubMed]
- Ma X, Kong Q, Wang C, Rajah G, Ding YC, Zhang YR, Du XY. Predicting asymptomatic coronary artery stenosis by aortic arch plaque in acute ischemic cerebrovascular disease: beyond the cervicocephalic atherosclerosis? Chin Med J (Engl) 2019;132:905-13. [Crossref] [PubMed]
- Liu S, Zhang Z, Liu B, Zhou S, Xie J, Han R, Kai S. One-step integrated coronary-carotid-cerebral computed tomography angiography to evaluate cardiovascular and cerebrovascular atherosclerosis. BMC Cardiovasc Disord 2023;23:367. [Crossref] [PubMed]
- Guo R, Deng J, Rong P, Zhou W, Zhang G, Peng S, Liang Q, Yang X, Hu P. One-stop combined CT angiography of coronary and craniocervical arteries: recommended as the first examination for patients suspected of coronary or craniocervical artery disease. Eur Radiol 2023;33:7034-43. [Crossref] [PubMed]
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41. [Crossref] [PubMed]
- Kong Q, Ma X, Li L, Wang C, Du X, Wan Y. Atherosclerosis Burden of Brain- and Heart-Supplying Arteries and the Relationship With Vascular Risk in Patients With Ischemic Stroke. J Am Heart Assoc 2023;12:e029505. [Crossref] [PubMed]
- Choi E, Byun E, Kwon SU, Kim N, Suh CH, Kwon H, Han Y, Kwon TW, Cho YP. Carotid Plaque Composition Assessed by CT Predicts Subsequent Cardiovascular Events among Subjects with Carotid Stenosis. AJNR Am J Neuroradiol 2021;42:2199-206. [Crossref] [PubMed]
- Dong J, Ma X, Qie J, Ji X. Aortic Complex Plaque Predicts the Risk of Cryptogenic Ischemic Cerebrovascular Disease Recurrence. Aging Dis 2016;7:114-20. [Crossref] [PubMed]
- Chatzikonstantinou A, Ebert AD, Schoenberg SO, Hennerici MG, Henzler T. Atherosclerosis in intracranial, extracranial, and coronary arteries with aortic plaques in patients with ischemic stroke of undetermined etiology. Int J Neurosci 2015;125:663-70. [Crossref] [PubMed]
- de Weert TT, Cretier S, Groen HC, Homburg P, Cakir H, Wentzel JJ, Dippel DW, van der Lugt A. Atherosclerotic plaque surface morphology in the carotid bifurcation assessed with multidetector computed tomography angiography. Stroke 2009;40:1334-40. [Crossref] [PubMed]
- Calvet D, Touzé E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation 2010;121:1623-9. [Crossref] [PubMed]
- Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, Eliasziw M. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-53. [Crossref] [PubMed]
- Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, Cabrejo L, Meseguer E, Guidoux C, Adraï V, Ratani S, Kusmierek J, Lapergue B, Klein IF, Gongora-Rivera F, Jaramillo A, Mazighi M, Touboul PJ, Steg PG. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke 2011;42:22-9. [Crossref] [PubMed]
- Hur J, Lee KH, Hong SR, Suh YJ, Hong YJ, Lee HJ, Kim YJ, Lee HS, Chang HJ, Choi BW. Prognostic value of coronary computed tomography angiography in stroke patients. Atherosclerosis 2015;238:271-7. [Crossref] [PubMed]
- Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, Taubert KA. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation 2003;108:1278-90. [Crossref] [PubMed]
- Hoshino A, Nakamura T, Enomoto S, Kawahito H, Kurata H, Nakahara Y, Ijichi T. Prevalence of coronary artery disease in Japanese patients with cerebral infarction: impact of metabolic syndrome and intracranial large artery atherosclerosis. Circ J 2008;72:404-8. [Crossref] [PubMed]
- Gupta A, Mtui EE, Baradaran H, Salama G, Pandya A, Kamel H, Giambrone A, Sanelli PC. CT angiographic features of symptom-producing plaque in moderate-grade carotid artery stenosis. AJNR Am J Neuroradiol 2015;36:349-54. [Crossref] [PubMed]
- Kallikazaros IE, Tsioufis CP, Stefanadis CI, Pitsavos CE, Toutouzas PK. Closed relation between carotid and ascending aortic atherosclerosis in cardiac patients. Circulation 2000;102:III263-8. [Crossref] [PubMed]
- Parthenakis FI, Kochiadakis GE, Skalidis EI, Kanakaraki MK, Mezilis NE, Kanoupakis EM, Vardas PE, Nihoyannopoulos P. Aortic atherosclerotic lesions in the thoracic aorta detected by multiplane transesophageal echocardiography as a predictor of coronary artery disease in elderly patients. Clin Cardiol 2000;23:734-9. [Crossref] [PubMed]
- Fujita S, Sugioka K, Matsumura Y, Ito A, Hozumi T, Hasegawa T, Hanatani A, Naruko T, Ueda M, Yoshiyama M. Impact of concomitant coronary artery disease on atherosclerotic plaques in the aortic arch in patients with severe aortic stenosis. Clin Cardiol 2013;36:352-7. [Crossref] [PubMed]
- Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S. Aortic arch plaques and risk of recurrent stroke and death. Circulation 2009;119:2376-82. [Crossref] [PubMed]