
Aneurysm, meningitis, and subarachnoid hemorrhage secondary to intracranial Aspergillosis in an immunocompetent patient: a case description
Introduction
Aspergillus, a globally pervasive group of molds, encompasses over 100 distinct species. These organisms are characterized as opportunistic pathogens, with Aspergillus fumigatus being the primary agent of human infections within this genus (1). Infections of the central nervous system (CNS) by Aspergillus are relatively rare, constituting merely 5–10% of all CNS fungal infections. They typically appear in immunocompromised patients receiving chemotherapy, transplantation, or immune-modulating therapies but also in immunocompetent individuals (2). The involvement of the CNS is usually caused by the dissemination of Aspergillus from a primary site outside of the CNS or occurs after invasive procedures such as neurosurgical or vascular intervention (1). The mortality rate of Aspergillus infection is approximately 10% to 20% in the immunocompetent persons but can reach to 85% to 100% in immunocompromised patients (3-5).
Clinical manifestations of Aspergillus infection in the CNS are heterogeneous, ranging from headaches and seizures to focal neurological deficits often resulting from stroke. In immunocompromised individuals, there is typically a notable history of immune dysfunction, and Aspergillus infections frequently affect multiple body sites. In contrast, immunocompetent patients often present with isolated brain lesions due to CNS aspergillosis, posing significant diagnostic challenges (6). Diagnosing invasive aspergillosis is complicated by the nonspecific nature of its clinical symptoms and the low detection rate of Aspergillus in cerebrospinal fluid (CSF) (7). Consequently, Aspergillus infections are prone to being misdiagnosed and undertreated in the immunocompetent population. The primary clinical diagnostic approaches for Aspergillus infection have traditionally relied on brain biopsies; autopsies; pathological examination of adjacent brain tissues, CSF, or tissue culture; and metagenomics next-generation sequencing (mNGS) of the CSF (8). While tissue biopsy is an invasive and often impractical method, mNGS offers a rapid, cost-effective, and unbiased approach for screening a wide array of pathogens in CSF with a single test (9). The use of mNGS significantly enhances the detection rate of atypical pathogens and broadens our understanding of the clinical presentation of Aspergillus infections in the CNS. In this report, we present a case of CNS aspergillosis in an immunocompetent woman. Her clinical presentation included symptoms of meningitis, a cerebral aneurysm, and cerebral vasospasm resulting from subarachnoid hemorrhage (SAH).
Case presentation
A 54-year-old female patient was admitted to our neurology ward on November 14, 2022, complaining of an 8-day history of persistent fever and headache. Initially, she reported symptoms of fever, headache, dizziness, nausea, diarrhea, and vomiting. Upon examination, she was alert and demonstrated normal mental status. Cranial nerve examination yielded normal results. Motor function and sensory perception in her extremities were symmetrical, and her neck was supple without Kernig’s sign. A lumbar puncture was performed, revealing an intracranial pressure (ICP) of 273 mmH2O. The CSF appeared with a transparent, faint-yellow color and without clots. CSF analysis showed a white blood cell (WBC) count of 17 (normal range, 0–8)/µL, predominantly lymphocytes (94%) and monocytes (6%), with an absence of red blood cells (RBCs). No atypical cells were noted. The glucose level in the CSF was 3.32 mmol/L (serum glucose: 5.9 mmol/L), and the protein concentration was 30.63 mg/dL. CSF staining (India ink, acid-fast) and bacterial culture yielded negative results.
The initial brain magnetic resonance imaging (MRI), both plain and contrast-enhanced, conducted on November 11, 2022, did not reveal any parenchymal or meningeal lesions. However, it indicated slight swelling in the left temporal lobe and insula (Figure 1 and Figure S1). The WBC in the blood was 6.33 (normal range, 3.5–9.5)×109/L, and the neutrophil count was 3.80 (normal range, 1.8–6.3)×109/L. The mononuclear leucocyte count was 0.67 (normal range, 0.1–0.6)/pL in the serum, the monocyte percentage was 10.6 (normal range, 3–10), and the hemoglobin level was 104 (normal range, 115–150) g/L.

The sedimentation rate, liver and kidney function, coagulation function, procalcitonin level, tuberculosis antibody test, tuberculosis infection T-cell test, and G test were all negative. The serum galactomannan levels were 0.1093 (normal range, 0–0.5). The blood culture for both bacteria and fungi was negative.
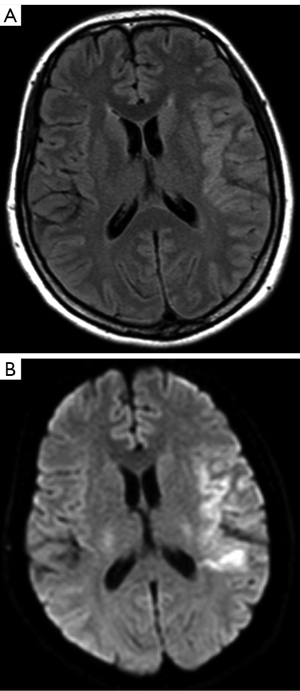
On the second day, she was admitted to the neurological disease ward and exhibited partial motor aphasia and hypomnesia. MRI was repeated on November 15, 2022. The left temporal lobe, insula, and the adjacent meninges appeared hyperintense on diffusion-weighted imaging (DWI) and T2-weighted fluid-attenuated inversion recovery (FLAIR) images and showed linear enhancement on the adjacent meninx on the contrast-enhanced MRI scan (Figure 2, Figure S2). These findings, combined the clinical symptoms, indicated a differential diagnosis of encephalopyosis and viral encephalitis. Ceftriaxone and acyclovir were prescribed.
The transcranial Doppler ultrasound was conducted on November 15, 2022, and indicated the following: (I) mild stenosis of the right subclavian artery; (II) increased flow velocity in the initiation of the right internal carotid artery; (III) moderate stenosis of the terminal segment of the bilateral internal carotid arteries and middle cerebral artery (MCA); (IV) moderate stenosis of the A1 section of the right arteria cerebral anterior; and (V) mild-to-moderate stenosis of the P2 section of the left posterior cerebral artery (PCA).
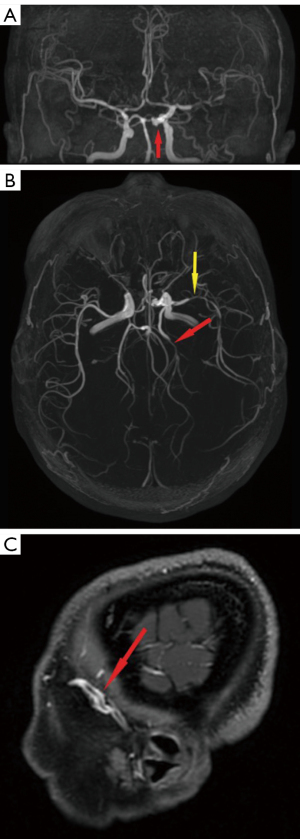
The contrast-enhanced scans of magnetic resonance angiography (MRA) and MRI of the brain were completed on November 17, 2022. Angiography revealed a staghorn-like aneurysm on the C5–C6 section of the left internal carotid artery (Figure 3A) and stenosis on the M2 section of the left MCA and P2 section of the left PCA (Figure 3B). Radiologically, lesions appeared enhanced in the meninx on the left temporal lobe on contrast-enhanced T1-weighted sequences (Figure S3A), and a swollen left temporal lobe was apparent on the T2-weighted FLAIR sequences (Figure S3B). The vessel wall of the M2 section of the left MCA showed homogeneous annular contrast enhancement on the contrast-enhanced T1-weighted sequences (Figure 3C).
The patient’s clinical symptoms, including the elevated WBC count and increased ICP in the CSF, along with the MRI findings, strongly suggested a CNS infection. Consequently, mNGS was conducted on both blood and CSF samples. Although the mNGS of the CSF showed no detectable pathogen, the blood analysis revealed 5,200 copies/mL of Aspergillus fumigatus. Based on these findings, an Aspergillus infection was diagnosed. Treatment with intravenous voriconazole commenced on November 16, 2022, at a dosage of 0.2 g twice daily.
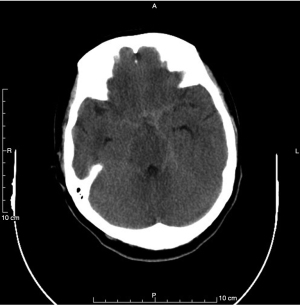
The patient felt explosive and intolerable headache on November 23, 2022. An urgent computed tomography (CT) scan of the head was arranged. The CT scan showed linear hyperdensity on the suprasellar cistern and bilateral tentorial area, which had prompted a SAH due to the rupture of the aneurysm on the left internal carotid (Figure 4). Nimodipine was pumped to resolve vasospasm. Digital subtraction angiography (DSA) showed a staghorn-like aneurysm on the C5–C6 section of the left internal carotid artery, and there was no obvious stenosis on the left MCA (Figure S4). The patient was transferred to the Neurosurgery Department to undergo the stent-assisted aneurysm embolization. She was prescribed tablets of voriconazole, aspirin, and clopidogrel after the surgery.
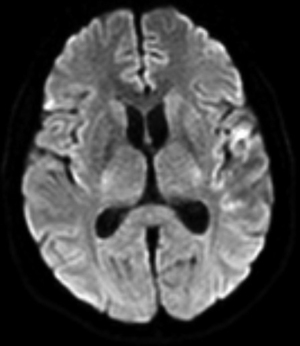
On December 26, 2022, the patient was readmitted to our neurological disease ward. The symptoms gradually improved, and a follow-up MRI confirmed radiological remission (Figure 5, Figure S5). The reexamination of mNGS of the blood showed 0 copy of Aspergillus fumigatus.
The plain and contrast-enhanced MRI was repeated on February 27, 2023, which indicated a significant recovery of lesion burden (Figure S6).
On March 7, 2023, the patient was readmitted to our neurology ward. Upon evaluation, she was alert with a normal mental status. The cranial nerve examination yielded ordinary results, and both motor function and sensory perception in her extremities were found to be symmetrical. Her neck was supple and negative for Kernig’s sign. She exhibited lingering partial motor aphasia and hypomnesia. Other nervous system signs were normal.
Lumbar puncture was repeated on March 9, 2023, and the ICP was 90 mmH2O. The CSF was transparent and without clots. The WBC count in the CSF was 2/µL, comprising 50% lymphocytes and 50% monocytes. There were no atypical cells. In the CSF, the concentration of sugar was 3.6 (normal range, 2.24–3.92) mmol/L (serum 5.4 mmol/L) while that of protein was 34.31 (normal range, 15–40) mg/dL. In subsequent analyses, the CSF continued to yield negative results for India ink staining, acid-fast staining, and microbial culture. mNGS of the blood and CSF revealed negative findings for Aspergillus. The liver function was normal, and the blood galactomannan antigen test produced negative results. The stenosis of the MCA and PCA was significantly alleviated according to brain MRA (Figure S7). The patient continued taking oral voriconazole tablets, 0.2 g each time, twice daily until June, 2023.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Aspergillus fumigatus is the most common pathogen of invasive aspergillosis. Acute, subacute, or chronic onset of aspergillosis can occur in the CNS (10). Typically, Aspergillus fumigatus infects immunodeficient or immunosuppressed patients, such as those positive for HIV. However, the patients described in this report was an immunocompetent middle-aged woman. She had been organizing ancient books 1 month prior to disease onset, and she was fatigued and stressed for work on those days. Therefore, we speculate that her infection might have been due to the inhalation or aspiration of fungal spores (11).
The pathophysiological mechanism underlying invasive aspergillosis primarily involves vascular invasion leading to subsequent tissue infarction and necrosis. CNS aspergillosis can arise through hematogenous dissemination from the bloodstream or via direct extension from adjacent structures such as the ear, paranasal sinuses, or mastoids, particularly in patients with localized invasive aspergillosis (12). A hallmark of intracranial aspergillosis is significant vascular invasion, often accompanied by secondary thrombosis or hemorrhage, a characteristic feature attributable to its angio-invasive nature. In our case, mNGS was positive for Aspergillus fumigatus DNA in the serum but negative in the CSF, and the bone near the location of the angioma was intact (Figure 4), which suggested that hematogenous dissemination was the most probable infection route. The Aspergillus fumigatus infection and hypha proliferation in the vessel walls led to the formation of aneurysm on the C5–C6 section of the left internal carotid aneurysm, causing subsequent SAH and cerebral vasospasm. The invasion of brain tissue via the cerebral vessels resulted in meningitis of the left temporal lobe, insula, and adjacent meninges. In patients with CNS aspergillosis, the most common clinical presentations are focal lesions or brain abscesses (13). Less frequently observed are cerebral infarctions, which may result from septic embolism, vascular thrombosis, mycotic aneurysms, or continuous invasion of cerebral tissue through granuloma formation (14). Instances of pure meningitis, without the involvement of other CNS structures, have been seldom reported in the context of CNS aspergillosis (10).
CNS aspergillosis lesions, based on radiological characteristics, can be categorized into two types: parenchymal lesions within the cerebral lobes and meningeal lesions in the meninges (8). Parenchymal lesions typically manifest as brain abscesses, are often located at the corticomedullary junction, and are characterized by ring enhancement in MRI scans. These lesions present with nonspecific, localization-dependent clinical symptoms. On the other hand, meningeal lesions are commonly found in the cavernous sinus, retro-orbital region, and frontotemporal areas. These lesions are frequently associated with severe cerebrovascular complications, such as infection-related cerebral aneurysms, vascular stenosis, cerebral infarction, and SAH (8). Meningitis due to Aspergillus can lead to vascular invasion, resulting in cerebrovascular aspergillosis or mycotic aneurysm formation. Infarctions, often accompanied by hemorrhage, are indicative of cerebral aspergillosis in cases of vessel invasion and detectable via CT. Depending on the specific CNS aspergillosis form, patients may exhibit symptoms such as headache, seizures, and altered sensorium, hemiparesis, or cranial nerve palsies (15).
As for the laboratory diagnosis of CNS aspergillosis, the positive rate of Aspergillus culture is limited. In one study, only one-third of the 92 patients with Aspergillus meningitis cultured positive (10). For the detection of fungal antigens, the galactomannan is nonspecific for Aspergillus, as variable amounts can be positive in several other fungi (16); β-D-glucan seems to be more sensitive, but it can also be detected in patients undergoing bacteremia or recipients of immunoglobulins or β-lactam antibiotics, showing high false positivity (17). In our case, the galactomannan antigen test could not be performed for the CSF due to limitations in local medical services. Stereotactic brain biopsy can be used for diagnostic and therapeutic purposes and is usually well tolerated. However, it is probably underutilized due to its invasiveness and the risk of hemorrhage (18). In this case, the patient refused to undergo the brain biopsy. Conventional mycological techniques such as culture and cytology are insensitive and time-consuming, and diagnostic CNS biopsies are regarded as too risky in severely ill patients (1).
Unlike traditional tests for specific microbes or categories of infection, mNGS of the CSF can screen for nearly all potential CNS infections and identify novel and unexpected pathogens (19). It has been shown to be helpful in supporting the exclusion of CNS infection when a coinfection is suspected in n immunosuppressed patients or when a noninfectious cause (such as autoimmune condition) is clinically favored (9). mNGS is fundamentally a direct-detection method and relies on the presence of nucleic acid from the causative pathogen in the sample. One multicenter, prospective study confirmed that compared to conventional methods, mNGS can identify more potential pathogens than can conventional direct-detection testing of CSF. Moreover, it can detect pathogens earlier and is more sensitive for CNS infections (20,21), with a sensitivity of up to 80% for cerebral aspergillosis. Therefore, Xing et al. speculated that mNGS may serve as a future, frontline diagnostic test for cerebral aspergillosis (21). However, mNGS data require detailed analysis to determine whether the identified microbes represent a true pathogen or environmental contamination (9). In cases where CNS infection is clinically suspected, it is advisable to use broad-spectrum gene sequencing technologies on both CSF and blood samples. This approach maximizes the likelihood of accurately identifying potential pathogens.
Treatment with antifungal agents for aspergillosis of the CNS is usually compromised by resistance and limited CNS penetration (1). Therefore, the Aspergillus infection of CNS usually progresses rapidly, thus contributing to high mortality rates (1,14). Surgical removal of fungal infested-tissues is challenging but usually effective, especially in Aspergillus. Neurosurgical assessment can remove necrotic areas that might contain viable fungi with low antifungal drug penetration (1). On the other hand, surgical procedures involving intracranial invasive aspergillosis may lead to severe complications owing to obvious or insidious angio-invasion at the time of surgery, including massive anaphylaxis, intracerebral hemorrhage and SAH, meningitis, hydrocephalus, widespread dissemination of the Aspergillus, and multiple infarcts. With severe or life-threatening complications, such as expanding lesions or hemorrhage, neurosurgical intervention may be required (14).
Voriconazole, along with alternatives such as liposomal amphotericin B and isavuconazole, is recommended as the first-line therapy for all forms of invasive aspergillosis. Voriconazole is favored due to its lower toxicity, superior CNS penetration, and broader spectrum of activity against fungal pathogens compared to amphotericin B (22). The prognosis of CNS Aspergillus infection tends to be more favorable in immunocompetent patients than in those who are immunocompromised. However, diagnosing Aspergillus infection in immunocompetent individuals is often more challenging. Once diagnosed, prompt administration of voriconazole in immunocompetent patients is critical for an advantageous prognosis. The American Society of Infectious Disease endorses voriconazole as the primary treatment for aspergillosis (9). The recommended duration of antifungal therapy is subject to debate and varies widely in the literature, with most institutions suggesting a treatment period of 6–18 months. This typically includes an initial phase of intravenous therapy followed by oral administration. Early diagnosis and persistence with antifungal treatment are crucial. The duration of therapy should be tailored to each patient, with the response to the medication, the lesion’s location, and the patient’s overall condition being taken into account. The relatively better outcomes observed in immunocompetent patients may be due to their more robust response to conventional medical therapy and a higher tolerance for aggressive surgical interventions compared to immunocompromised patients.
Following a 6-month course of voriconazole treatment, our patient experienced complete resolution of her headache. Concurrently, there was a gradual improvement in the affected brain parenchyma, and both the ICP and CSF WBC counts returned to normal levels. These clinical improvements further corroborated the diagnosis of CNS aspergillosis.
Conclusions
Immunocompetent individuals are also susceptible to Aspergillus infections. Notably, cerebrovascular diseases frequently accompany CNS aspergillosis, particularly in cases involving meningeal aspergillosis. In our case, the presence of Aspergillus fumigatus in the blood and the organism’s affinity for cerebral vessels likely played a pivotal role in the pathogenesis. Voriconazole has proven to be an effective treatment for CNS infections caused by Aspergillus. Therefore, the emphasis on early diagnosis and timely initiation of treatment is crucial to achieving positive patient outcomes.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1404/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol 2018;17:362-72. [Crossref] [PubMed]
- Schwartz S, Cornely OA, Hamed K, Marty FM, Maertens J, Rahav G, Herbrecht R, Heinz WJ. Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med Mycol 2020;58:417-24. [Crossref] [PubMed]
- Bokhari R, Baeesa S, Al-Maghrabi J, Madani T. Isolated cerebral aspergillosis in immunocompetent patients. World Neurosurg 2014;82:e325-33. [Crossref] [PubMed]
- Marzolf G, Sabou M, Lannes B, Cotton F, Meyronet D, Galanaud D, Cottier JP, Grand S, Desal H, Kreutz J, Schenck M, Meyer N, Schneider F, Dietemann JL, Koob M, Herbrecht R, Kremer S. Magnetic Resonance Imaging of Cerebral Aspergillosis: Imaging and Pathological Correlations. PLoS One 2016;11:e0152475. [Crossref] [PubMed]
- Spapen H, Spapen J, Taccone FS, Meersseman W, Rello J, Dimopoulos G, Charles PE, Rao R, Pérez M, Martin C, Vogelaers D, Blot SI. Cerebral aspergillosis in adult critically ill patients: a descriptive report of 10 patients from the AspICU cohort. Int J Antimicrob Agents 2014;43:165-9. [Crossref] [PubMed]
- Kumar D, Nepal P, Singh S, Ramanathan S, Khanna M, Sheoran R, Bansal SK, Patil S. CNS aspergilloma mimicking tumors: Review of CNS aspergillus infection imaging characteristics in the immunocompetent population. J Neuroradiol 2018;45:169-76. [Crossref] [PubMed]
- Lyons JL, Zhang SX. Current laboratory approaches to diagnosis of CNS fungal infections. Future Microbiol 2016;11:175-7. [Crossref] [PubMed]
- Ma Y, Li W, Ao R, Lan X, Li Y, Zhang J, Yu S. Central nervous system aspergillosis in immunocompetent patients: Case series and literature review. Medicine (Baltimore) 2020;99:e22911. [Crossref] [PubMed]
- Wilson MR, O'Donovan BD, Gelfand JM, Sample HA, Chow FC, Betjemann JP, et al. Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. JAMA Neurol 2018;75:947-55. [Crossref] [PubMed]
- Antinori S, Corbellino M, Meroni L, Resta F, Sollima S, Tonolini M, Tortorano AM, Milazzo L, Bello L, Furfaro E, Galli M, Viscoli C. Aspergillus meningitis: a rare clinical manifestation of central nervous system aspergillosis. Case report and review of 92 cases. J Infect 2013;66:218-38. [Crossref] [PubMed]
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016;30:229-46. [Crossref] [PubMed]
- Candoni A, Klimko N, Busca A, Di Blasi R, Shadrivova O, Cesaro S, et al. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses 2019;62:252-60. [Crossref] [PubMed]
- Kourkoumpetis TK, Desalermos A, Muhammed M, Mylonakis E. Central nervous system aspergillosis: a series of 14 cases from a general hospital and review of 123 cases from the literature. Medicine (Baltimore) 2012;91:328-36. [Crossref] [PubMed]
- McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med 2014;371:150-60. [Crossref] [PubMed]
- Ray S, Balaini N, Chakravarty K, Pattanayak S, Goel A, Takkar A, Lal V. Special scenarios in the management of central nervous system aspergillosis: a case series and review of literature. Postgrad Med J 2019;95:382-9. [Crossref] [PubMed]
- Barton RC. Laboratory diagnosis of invasive aspergillosis: from diagnosis to prediction of outcome. Scientifica (Cairo) 2013;2013:459405. [Crossref] [PubMed]
- Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:433-42. [Crossref] [PubMed]
- Economides MP, Ballester LY, Kumar VA, Jiang Y, Tarrand J, Prieto V, Torres HA, Kontoyiannis DP. Invasive mold infections of the central nervous system in patients with hematologic cancer or stem cell transplantation (2000-2016): Uncommon, with improved survival but still deadly often. J Infect 2017;75:572-80. [Crossref] [PubMed]
- Wilson MR, Shanbhag NM, Reid MJ, Singhal NS, Gelfand JM, Sample HA, Benkli B, O'Donovan BD, Ali IK, Keating MK, Dunnebacke TH, Wood MD, Bollen A, DeRisi JL. Diagnosing Balamuthia mandrillaris Encephalitis With Metagenomic Deep Sequencing. Ann Neurol 2015;78:722-30. [Crossref] [PubMed]
- Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N Engl J Med 2019;380:2327-40. [Crossref] [PubMed]
- Xing XW, Zhang JT, Ma YB, He MW, Yao GE, Wang W, Qi XK, Chen XY, Wu L, Wang XL, Huang YH, Du J, Wang HF, Wang RF, Yang F, Yu SY. Metagenomic Next-Generation Sequencing for Diagnosis of Infectious Encephalitis and Meningitis: A Large, Prospective Case Series of 213 Patients. Front Cell Infect Microbiol 2020;10:88. [Crossref] [PubMed]
- Góralska K, Blaszkowska J, Dzikowiec M. Neuroinfections caused by fungi. Infection 2018;46:443-59. [Crossref] [PubMed]


