
Clinical analysis of uterine parameters evaluated by preoperative magnetic resonance imaging in patients treated by hysteroscopic approach with previous cesarean scar defect-related abnormal uterine bleeding: a retrospective cohort study
Introduction
The cesarean delivery rate continues to rise globally, as reported by the World Health Organization (WHO) (1,2). In the past decade, although great efforts have been implemented to decrease the cesarean delivery rate in China, the overall national cesarean delivery rate is still high (3-6). It has been shown that inappropriate cesarean delivery is related to numerous short- and long-term complications in women, including bleeding, infection, pelvic adhesions, abnormal placentation, uterine rupture, still birth, preterm birth, and even hysterectomy in the subsequent pregnancy (7,8).
As one of the long-term complications, a previous cesarean scar defect (PCSD) is the presence of a triangular area in the myometrium of the anterior lower uterine wall at the site of the previous caesarean incision, of which the critical clinical symptoms are abnormal uterine bleeding (AUB), dysmenorrhea, chronic pelvic pain, and secondary infertility (9-11). The predominant clinical symptom of PCSD-related AUB is early-cycle intermenstrual bleeding or a protracted menstrual period following the cesarean delivery, which has a considerable detrimental impact on the quality of life of women (11-14). According to a recent systematic review (11), the prevalence of AUB in PCSD patients presenting for imaging for a gynecologic indication is 76.4%, and the mean menstrual duration in symptomatic patients with PCSD is as long as 13.4 days.
Hysteroscopic surgery for PCSD involves the removal of the superior and inferior edge of the uterine scar defect to facilitate the drainage of menstrual blood and fulguration of the bottom of the defect to prevent blood production in the meantime (15-17). In our previous study (18), the clinical efficacy of PCSD-related AUB with hysteroscopic treatment was 78.8%, but the clinical cure rate was only 24.2%. It seems that not all of the patients were ideal candidates for the surgery.
Studies on magnetic resonance imaging (MRI) for PCSD diagnosis and prognosis have rarely been reported to date. The aim of this study was to analyze the factors that might have an impact on the clinical cure rate of patients with PCSD-related AUB following hysteroscopic treatment as assessed by preoperative MRI. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1205/rc).
Methods
Patients
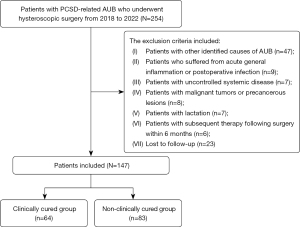
All 254 patients (N=254) with PCSD-related AUB who underwent hysteroscopic surgery in the Gynecology Department of Third Xiangya Hospital of Central South University from 2018 to 2022 were retrospectively recruited for this study. The inclusion criteria for this study included the following: (I) patients with prolonged menstrual duration of at least 3 months following their cesarean delivery; (II) normal ovarian function and ovulation; (III) PCSD was diagnosed by hysteroscopy. The exclusion criteria were as follows: (I) patients with other identified causes of AUB, such as endometrial polyps, adenomyosis, and ovulation disorder; (II) patients who had experienced acute general inflammation or postoperative infection; (III) patients with uncontrolled systemic disease; (IV) patients with malignant tumors or precancerous lesions; (V) patients who were lactating; (VI) patients who had undergone subsequent therapy following surgery within 6 months, such as hormone therapy; (VII) lost to follow-up. A total of 147 patients (n=147) were identified according to the above criteria. A flow chart of detailed participant selection is presented in Figure 1.
Ethical statement
The protocol of this study was approved by the Ethics Committee of the Third Xiangya Hospital of Central South University (No. 2020-S625). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients involved in this study received diagnosis and treatment according to standard procedures. All patients were fully informed of surgical procedures, the benefits, potential risks, and outcomes of the treatment before the surgery. All participants provided informed consent.
MRI examination
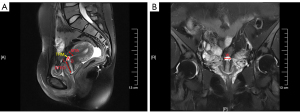
All participants underwent MRI (Ingenia 3.0T; Philips Co., Eindhoven, Netherlands) evaluation by T2-weighted images (T2WI) pre-operation during the middle to late luteal phase to reduce vaginal bleeding interference. The imaging protocol comprised a T2WI fat saturation sequence (sagittal and coronal planes, repetition time 4,000 ms, echo time 79 ms, slice thickness 5 mm, spacing 1.5 mm, acquisition time 1 min and 32 s). The parameters, involving the length, width, depth, thickness of the remaining muscle layer of the defect, myometrial thickness adjacent to the defect, and distance of the defect to the cervix external oral, were measured (detailed description in Figure 2) by the same 2 chief radiologists. If the measurement difference was greater than 2 mm, the senior radiologist would review the measurement. Length, depth, thickness of the remaining muscle layer of the defect, myometrial thickness adjacent to the defect, and distance of the defect to the external cervical os were measured in the sagittal plane, and the coronal plane was only used for width.
Hysteroscopic surgery procedures
All the participants underwent hysteroscopic surgery within 1 week after menstruation. Hysteroscopy was performed by a surgeon using a bipolar resectoscope (KARL STORZ SE & Co., Tuttlingen, Germany) with saline solution as the medium of distension. The scar tissue of the defect’s inferior edge was removed until the wall was continuous with the cervical canal. The endometrium at the bottom of the diverticulum was electrocauterized afterwards. The operation was conducted under visual examination to make sure to achieve a complete hemostasis.
Follow-up method
All the participants were followed up 6 months post-operation. The data were collected from medical records and telephone interviews, including patients’ age, clinical manifestations, gravidity and parity, history of cesarean section deliveries, MRI parameters (including the length, width, depth, thickness of the remaining muscle layer of the defect, myometrial thickness adjacent to the defect, and distance of the defect to the external cervical os), and duration of postoperative menstruation. The clinical efficacy of postoperative menstruation was assessed by clinical cure, improvement, and ineffectiveness. Clinical cure was defined as no postmenstrual spotting after the surgery. Improvement was defined as shortened postmenstrual spotting post-operation. Ineffectiveness was defined as no obvious change in menstruation after the surgery. Based on the duration of menstruation after surgery, the participants were divided into clinically cured and non-clinically cured (improvement or ineffectiveness) groups.
Statistical analysis
Statistical analysis was performed with the software Statistical Analysis System 9.4 (SAS Institute, Cary, NC, USA). The 2-sided chi-square test was used to analyze categorical data, which were expressed as frequency and percentage in each group. A 2-sided Student’s t-test was used to analyze normally distributed data, which were presented as mean ± standard deviation (SD). The continuous variables of MRI parameters before the surgery are divided by median. A logistic regression analysis was applied to determine the dominant variables. A 2-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
There were 64 clinically cured (43.5%) and 83 non-clinically cured (56.5%) patients comprising a total of 147 patients involved in the study. Table 1 lists the 147 individuals’ characteristics in detail. No significant differences were detected in age, menstrual duration before surgery, gravidity, parity, number of cesarean sections, or time since the previous cesarean section in the 2 groups. The menstrual duration after hysteroscopic surgery was improved in the clinically cured group (6.3 days), which was significantly shorter than the 12.1 days in the non-clinically cured group (P<0.001, Table 1). The time since AUB had appeared was significantly correlated with the clinical cure rate (P=0.028, Table 1), since the symptom lasted less time in the clinically cured group (49.7 months) than in the non-clinically cured group (62.6 months).
Table 1
| Variables | Clinically cured group (n=64) | Non-clinically cured group (n=83) | P value |
|---|---|---|---|
| Age (years, mean ± SD) | 34.5±3.8 | 35.1±4.0 | 0.184 |
| Menstrual duration before surgery (days, mean ± SD) | 14.1±2.7 | 14.5±2.5 | 0.148 |
| Gravidity (n, %) | |||
| 1 | 17 (26.6) | 17 (20.5) | 0.405 |
| 2 | 21 (32.8) | 36 (43.4) | |
| ≥3 | 26 (40.6) | 30 (36.1) | |
| Parity (n, %) | |||
| 1 | 31 (48.4) | 35 (42.2) | 0.505 |
| ≥2 | 33 (51.6) | 48 (57.8) | |
| Number of cesarean section (n, %) | |||
| 1 | 33 (51.6) | 38 (45.8) | 0.510 |
| ≥2 | 31 (48.4) | 45 (54.2) | |
| Time since the previous cesarean section (months, mean ± SD) | 74.2±36.6 | 76.3±45.6 | 0.379 |
| Time since AUB appeared (months, mean ± SD) | 49.7±35.0 | 62.6±44.2 | 0.028 |
| Menstrual duration after surgery (days, mean ± SD) | 6.3±1.2 | 12.1±2.8 | <0.001 |
SD, standard deviation; AUB, abnormal uterine bleeding.
MRI parameters
Table 2 displays the T2-weighted MRI parameters of the defect before the surgery. No significant differences were shown in the uterus position, the width, depth, or thickness of the remaining muscle layer of the defect before hysteroscopic surgery. The length of the defect (P=0.045), myometrial thickness adjacent to the defect (P=0.019), and distance from the defect to the external cervical os (P=0.013) were significantly correlated with the clinical cure rate post-operation (Table 2).
Table 2
| Variables | Clinically cured group (n=64) | Non-clinically cured group (n=83) | P value |
|---|---|---|---|
| Uterus position (n, %) | |||
| Anteflexed | 20 (31.3) | 34 (41.0) | 0.226 |
| Retroflexed | 44 (68.8) | 49 (59.0) | |
| Length of defect (mm, n, %) | |||
| <8 | 36 (56.3) | 32 (38.6) | 0.045 |
| ≥8 | 28 (43.8) | 51 (61.4) | |
| Width of defect (mm, n, %) | |||
| <17 | 30 (46.9) | 45 (54.2) | 0.409 |
| ≥17 | 34 (53.1) | 38 (45.8) | |
| Depth of defect (mm, n, %) | |||
| <6 | 29 (45.3) | 37 (44.6) | 0.999 |
| ≥6 | 35 (54.7) | 46 (55.4) | |
| Thickness of the remaining muscle layer of defect (mm, n, %) | |||
| <3 | 29 (45.3) | 31 (37.3) | 0.398 |
| ≥3 | 35 (54.7) | 52 (62.7) | |
| Myometrial thickness adjacent to the defect (mm, n, %) | |||
| <11 | 43 (67.2) | 39 (47.0) | 0.019 |
| ≥11 | 21 (32.8) | 44 (53.0) | |
| Distance from the defect to the external cervical os (mm, n, %) | |||
| <30 | 38 (59.4) | 32 (38.6) | 0.013 |
| ≥30 | 26 (40.6) | 51 (61.4) | |
MRI, magnetic resonance imaging.
Risk factors for PCSD prognosis
Tables 3 and 4 list univariate and multivariate logistic regression used to examine potential influences on the prognosis for PCSD following hysteroscopic surgery. Table 3 shows the results of the univariate logistic regression analysis of the clinical cure rate risk factors. Compared with the clinically cured group, the length of the defect [P=0.034, odds ratio (OR) =2.049, 95% confidence interval (CI): 1.061–4.007], myometrial thickness adjacent to the defect (P=0.015, OR =2.310, 95% CI: 1.184–4.601), and distance from the defect to the external cervical os (P=0.013, OR =2.329, 95% CI: 1.203–4.578) displayed in the T2-weighted MRI parameters before the surgery are greater in the non-clinically cured group. The larger the length of the defect (≥8 mm), the myometrial thickness adjacent to the defect (≥11 mm), and the distance from the defect to the external cervical os (≥30 mm), the worse the clinical cure rate. There were no significant differences in other variables between the 2 groups (P>0.05).
Table 3
| Variables | Estimate | SE | Wald | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.039 | 0.043 | 0.820 | 0.365 | 1.040 | 0.956–1.132 |
| Gravidity | ||||||
| 1 | ||||||
| 2 | 0.539 | 0.439 | 1.506 | 0.220 | 1.714 | 0.725–4.092 |
| ≥3 | 0.143 | 0.435 | 0.108 | 0.742 | 1.154 | 0.490–2.722 |
| Parity | ||||||
| 1 | ||||||
| ≥2 | 0.253 | 0.335 | 0.573 | 0.449 | 1.288 | 0.669–2.490 |
| Number of cesarean sections | ||||||
| 1 | ||||||
| ≥2 | 0.232 | 0.333 | 0.483 | 0.487 | 1.261 | 0.656–2.431 |
| Time since the last cesarean section | 0.001 | 0.004 | 0.119 | 0.730 | 1.001 | 0.993–1.009 |
| Time since AUB appeared | 0.008 | 0.004 | 3.516 | 0.061 | 1.008 | 0.999–1.017 |
| Uterus position | ||||||
| Anteflexed | ||||||
| Retroflexed | −0.423 | 0.350 | 1.459 | 0.227 | 0.655 | 0.326–1.293 |
| Length of defect (mm) | ||||||
| <8 | ||||||
| ≥8 | 0.717 | 0.338 | 4.503 | 0.034 | 2.049 | 1.061–4.007 |
| Width of defect (mm) | ||||||
| <17 | ||||||
| ≥17 | −0.294 | 0.334 | 0.778 | 0.378 | 0.745 | 0.386–1.431 |
| Depth of defect (mm) | ||||||
| <6 | ||||||
| ≥6 | 0.030 | 0.334 | 0.008 | 0.929 | 1.030 | 0.534–1.986 |
| Thickness of the remaining muscle layer of defect (mm) | ||||||
| <3 | ||||||
| ≥3 | 0.329 | 0.338 | 0.946 | 0.331 | 1.390 | 0.716–2.707 |
| Myometrial thickness adjacent to the defect (mm) | ||||||
| <11 | ||||||
| ≥11 | 0.837 | 0.345 | 5.881 | 0.015 | 2.310 | 1.184–4.601 |
| Distance from the defect to the external cervical os (mm) | ||||||
| <30 | ||||||
| ≥30 | 0.846 | 0.340 | 6.185 | 0.013 | 2.329 | 1.203–4.578 |
SE, standard error; OR, odds ratio; CI, confidence interval; AUB, abnormal uterine bleeding.
Table 4
| Variables | Estimate | SE | Wald | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Intercept | −0.946 | 0.383 | 6.111 | 0.014 | 0.388 | 0.179–0.808 |
| Time since AUB appeared (months) | 0.008 | 0.004 | 3.614 | 0.057 | 1.009 | 1.000–1.018 |
| Myometrial thickness adjacent to the defect (≥11 mm) | 0.740 | 0.357 | 4.297 | 0.038 | 2.095 | 1.047–4.261 |
| Distance from the defect to the external cervical os (≥30 mm) | 0.813 | 0.352 | 5.322 | 0.021 | 2.254 | 1.136–4.540 |
SE, standard error; OR, odds ratio; CI, confidence interval; AUB, abnormal uterine bleeding.
As displayed in Table 4, a multivariate logistic regression was examined using the meaningful variables from the univariate analysis. The data showed that myometrial thickness adjacent to the defect (P=0.038, OR =2.095, 95% CI: 1.047–4.261) and distance from the defect to the external cervical os (P=0.021, OR =2.254, 95% CI: 1.136–4.540) are risk factors for the clinical cure rate.
Discussion
As one of the long-term complications of cesarean delivery, the main clinical symptoms of PCSD are AUB, dysmenorrhea, chronic pelvic pain, and secondary infertility, according to previous literature (16,19). In various studies, the increasing number of cesarean deliveries, prolonged duration of active labor, single-layer myometrium closure without endometrial suture, and infection of the wound have been identified as risk factors for PCSD (11,20,21). In addition, maternal body mass index and gestational diabetes are also associated with PCSD (16,21,22). Postmenstrual spotting or prolonged menstrual duration is the predominant clinical symptom of PCSD-related AUB in patients (11,22), of which the mechanism is still unclear. The collection of menstrual blood in the uterine defect, resulting from the lack of muscle contractility around the scar and the poor drainage of menstrual flow through the fibrotic tissue below the niche, may explain the mechanism of PCSD-related AUB (11,23,24). Furthermore, retention of blood inside the uterine scar defect can also originate from endometriotic tissue due to inflammation, neovascularization, or adenomyosis (11,23-26).
Multiple surgical treatments have been applied to patients with PCSD-related AUB, including hysteroscopic, laparoscopic, and transvaginal surgery (16). Laparoscopic surgery could eliminate the uterine scar defect and strengthen the uterine wall at the same time (24). In addition, laparoscopic surgery could allow surgeons to explore the pelvis and remove adhesions (16,24). Different from the laparoscopic approach, hysteroscopic surgery for PCSD could correct the scar defect instead of strengthening the uterine wall (24,27). However, hysteroscopy is a minimally invasive procedure with similar clinical efficacy and less operative time, hospitalization time, expenses, and intra-operative blood loss than laparoscopy, as we previously reported (18). Some investigators have suggested that a hysteroscopic approach should not be applied when residual myometrium thickness is less than 3 mm on account of the increasing risk of uterine perforation, bladder injury, and uterine rupture in the subsequent delivery (23,24). However, previous studies have shown that the residual myometrium thickness value may be invariable or even increase after hysteroscopic PCSD surgery due to reduced internal pressure from the residue of menstrual fluid and the removal of inflammatory material (23,28). As a result of the careful operation, there were no severe complications in this patient cohort, such as perforation of the uterus, bladder injury, or postoperative hemorrhage.
So far, there are no standard guidelines for the treatment of PCSD-related AUB, especially regarding how to select an appropriate technique. In our previous study (18), the clinical efficacy of AUB after hysteroscopic surgery was 78.8%, but the clinical cure rate was only 24.2%. This can be explained by some of these patients being poorer candidates for hysteroscopic surgery. In this study, 43.5% of all the participants (n=64) with no more postmenstrual spotting after the surgery were classified as clinically cured. Simultaneously, 56.5% of the patients (n=83) showed shortened postmenstrual spotting or no obvious change in menstruation, who were classified within the non-clinically cured group. This raises the question of what the crucial factor for the prognosis of PCSD-related AUB patients could be.
In general, transvaginal ultrasonography (TVS) is the first suggested method for PCSD evaluation due to its effectiveness and affordability (20,29). A TVS standardized guideline (a modified Delphi procedure) could be applied for detailed PCSD evaluation in non-pregnant women (30,31). However, MRI is able to scan larger areas of the pelvis for further scrutiny, so one of its advantages is a more accurate and clear view in the pre-operation evaluation. Besides, MRI is operator-independent and could provide reproducible measurements for gynecologists (32).
Using logistic regression models, we discovered that the myometrial thickness adjacent to the defect and the distance from the defect to the external cervical os in preoperative MRI evaluation are risk factors for clinical cure rate. It seems that the time since the appearance of AUB and the length of the defect are also correlated with the outcomes of surgery. The cause could be related to the fact that when the cesarean delivery site is too high, different myometrium recovery at the upper and lower segments of the uterine incision will lead to a greater difference in the thickness of the upper and lower myometrium of the incision, which will affect the healing of the uterine incision and lead to a larger diverticulum formation (33,34). In the meantime, the longer the time since the appearance of AUB, the increased formation of scar tissue around the scar, which leads to poor contractility of uterine muscles around the cesarean scar over time. As the length of the defect increases, it has a greater chance of having a larger diverticulum. According to a prior study, longer menstrual bleeding is associated with a deeper and larger MRI abnormality (35). The key point of hysteroscopic surgery is the removal of the inferior edge of the uterine scar defect and fulguration of the bottom of the defect in the meantime. Persistent post-surgical AUB may be due to abundant endometrium within the diverticulum, which makes it difficult to perform electrocoagulation. However, the specific mechanism still needs to be further studied.
In this study, we, for the first time, applied MRI to PCSD patients and examined the impact of several variables on the prognosis of PCSD patients undergoing hysteroscopic surgery. With high measuring precision, the MRI could scan the pelvic area from different perspectives to evaluate the defect’s position, size, depth, shape, and relationship with surrounding tissues. In the meantime, the defect image of an MRI could be assessed by the surgeon without operating the imaging machine, as the TVS. Therefore, preoperative MRI evaluation is probably of great reference value in clinical cure rate prediction. Based on our research, the myometrial thickness adjacent to the defect and the distance from the defect to the external cervical os are 2 crucial indicators in preoperative MRI evaluation that are likely to become important indices in the choice of treatment. Our research shows that preoperative MRI evaluation may become one of the most valuable assessment methods for surgery method selection and clinical cure rate prediction.
As our study was retrospective in design, one of its limitations is that more cases and stratifications based on the basic characteristics of patients are required to provide more information.
Conclusions
The myometrial thickness adjacent to the defect and the distance from the defect to the external cervical os in preoperative MRI are risk factors for clinical cure rate in patients with PCSD-related AUB after hysteroscopic treatment, which is helpful for evaluating the prognosis of disease.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1205/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1205/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol of this study was approved by the Ethics Committee of the Third Xiangya Hospital of Central South University (No. 2020-S625). All participants provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Betran AP, Torloni MR, Zhang JJ, Gülmezoglu AMWHO Working Group on Caesarean Section. WHO Statement on Caesarean Section Rates. BJOG 2016;123:667-70. [Crossref] [PubMed]
- Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health 2021;6:e005671. [Crossref] [PubMed]
- Song C, Xu Y, Ding Y, Zhang Y, Liu N, Li L, Li Z, Du J, You H, Ma H, Jin G, Wang X, Shen H, Lin Y, Jiang X, Hu Z. The rates and medical necessity of cesarean delivery in China, 2012-2019: an inspiration from Jiangsu. BMC Med 2021;19:14. [Crossref] [PubMed]
- Liang J, Mu Y, Li X, Tang W, Wang Y, Liu Z, Huang X, Scherpbier RW, Guo S, Li M, Dai L, Deng K, Deng C, Li Q, Kang L, Zhu J, Ronsmans C. Relaxation of the one child policy and trends in caesarean section rates and birth outcomes in China between 2012 and 2016: observational study of nearly seven million health facility births. BMJ 2018;360:k817. [Crossref] [PubMed]
- Li HT, Hellerstein S, Zhou YB, Liu JM, Blustein J. Trends in Cesarean Delivery Rates in China, 2008-2018. JAMA 2020;323:89-91. [Crossref] [PubMed]
- Li HT, Luo S, Trasande L, Hellerstein S, Kang C, Li JX, Zhang Y, Liu JM, Blustein J. Geographic Variations and Temporal Trends in Cesarean Delivery Rates in China, 2008-2014. JAMA 2017;317:69-76. [Crossref] [PubMed]
- Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS Med 2018;15:e1002494. [Crossref] [PubMed]
- Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, Gibbons D, Kelly NM, Kennedy HP, Kidanto H, Taylor P, Temmerman M. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018;392:1349-57. [Crossref] [PubMed]
- Schepker N, Garcia-Rocha GJ, von Versen-Höynck F, Hillemanns P, Schippert C. Clinical diagnosis and therapy of uterine scar defects after caesarean section in non-pregnant women. Arch Gynecol Obstet 2015;291:1417-23. [Crossref] [PubMed]
- van der Voet LF, Bij de Vaate AM, Veersema S, Brölmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG 2014;121:236-44. [Crossref] [PubMed]
- Murji A, Sanders AP, Monteiro I, Haiderbhai S, Matelski J, Walsh C, Abbott JA, Munro MG, Maheux-Lacroix SInternational Federation of Gynecology and Obstetrics (FIGO) Committee on Menstrual Disorders and Related Health Impacts. Cesarean scar defects and abnormal uterine bleeding: a systematic review and meta-analysis. Fertil Steril 2022;118:758-66. [Crossref] [PubMed]
- Stegwee SI, Beij A, de Leeuw RA, Mokkink LB, van der Voet LF, Huirne JAF. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res 2020;29:1013-25. [Crossref] [PubMed]
- Chen H, Wang Y, Zhang H, Wang X. Vaginal repair of cesarean section scar defects: Preoperative hysteroscopic evaluation. Acta Obstet Gynecol Scand 2022;101:1308-14. [Crossref] [PubMed]
- Bij de Vaate AJ, Brölmann HA, van der Voet LF, van der Slikke JW, Veersema S, Huirne JA. Ultrasound evaluation of the Cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol 2011;37:93-9. [Crossref] [PubMed]
- Raimondo G, Grifone G, Raimondo D, Seracchioli R, Scambia G, Masciullo V. Hysteroscopic treatment of symptomatic cesarean-induced isthmocele: a prospective study. J Minim Invasive Gynecol 2015;22:297-301. [Crossref] [PubMed]
- Donnez O. Cesarean scar defects: management of an iatrogenic pathology whose prevalence has dramatically increased. Fertil Steril 2020;113:704-16. [Crossref] [PubMed]
- Feng YL, Li MX, Liang XQ, Li XM. Hysteroscopic treatment of postcesarean scar defect. J Minim Invasive Gynecol 2012;19:498-502. [Crossref] [PubMed]
- Zhang Q, Lei L, Zhang A, Zou L, Xu D. Comparative effectiveness of laparoscopic versus hysteroscopic approach in patients with previous cesarean scar defect: a retrospective cohort study. Ann Transl Med 2021;9:1529. [Crossref] [PubMed]
- Allornuvor GF, Xue M, Zhu X, Xu D. The definition, aetiology, presentation, diagnosis and management of previous caesarean scar defects. J Obstet Gynaecol 2013;33:759-63. [Crossref] [PubMed]
- Bij de Vaate AJ, van der Voet LF, Naji O, Witmer M, Veersema S, Brölmann HA, Bourne T, Huirne JA. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol 2014;43:372-82. [Crossref] [PubMed]
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, Tomás EI, Staff SM. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol 2018;219:458.e1-8. [Crossref] [PubMed]
- de Luget CD, Becchis E, Fernandez H, Donnez O, Quarello E. Can uterine niche be prevented? J Gynecol Obstet Hum Reprod 2022;51:102299. [Crossref] [PubMed]
- Tanimura S, Funamoto H, Hosono T, Shitano Y, Nakashima M, Ametani Y, Nakano T. New diagnostic criteria and operative strategy for cesarean scar syndrome: Endoscopic repair for secondary infertility caused by cesarean scar defect. J Obstet Gynaecol Res 2015;41:1363-9. [Crossref] [PubMed]
- Donnez O, Donnez J, Orellana R, Dolmans MM. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil Steril 2017;107:289-296.e2. [Crossref] [PubMed]
- Morris H. Surgical pathology of the lower uterine segment caesarean section scar: is the scar a source of clinical symptoms? Int J Gynecol Pathol 1995;14:16-20. [Crossref] [PubMed]
- Chen YY, Tsai CC, Kung FT, Lan KC, Ou YC. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwan J Obstet Gynecol 2019;58:541-4. [Crossref] [PubMed]
- Api M. Author's Response: Which cesarean scar defect should be treated; by which technique and by whom? J Minim Invasive Gynecol 2016;23:843-4. [Crossref] [PubMed]
- Tsuji S, Kimura F, Yamanaka A, Hanada T, Hirata K, Takebayashi A, Takashima A, Seko-Nitta A, Murakami T. Impact of hysteroscopic surgery for isthmocele associated with cesarean scar syndrome. J Obstet Gynaecol Res 2018;44:43-8. [Crossref] [PubMed]
- Jordans IPM, de Leeuw RL, Stegwee SI, Amso NN, Barri Soldevila PN, van den Bosch T, Bourne T, Brölmann HAM, Donnez O, Dueholm M, Hehenkamp WJK, Jastrow N, Jurkovic D, Mashiach R, Naji O, Streuli I, Timmerman D, van der Voet LF, Huirne JAF. Niche definition and guidance for detailed niche evaluation. Acta Obstet Gynecol Scand 2019;98:1351-2. [Crossref] [PubMed]
- Jordans IPM, de Leeuw RA, Stegwee SI, Amso NN, Barri-Soldevila PN, van den Bosch T, Bourne T, Brölmann HAM, Donnez O, Dueholm M, Hehenkamp WJK, Jastrow N, Jurkovic D, Mashiach R, Naji O, Streuli I, Timmerman D, van der Voet LF, Huirne JAF. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol 2019;53:107-15. [Crossref] [PubMed]
- Verberkt C, Jordans IPM, Van den Bosch T, Timmerman D, Bourne T, de Leeuw RA, Huirne JAF. How to perform standardized sonographic examination of uterine niche in non-pregnant women. Ultrasound Obstet Gynecol 2022;60:420-4. [Crossref] [PubMed]
- Fiocchi F, Petrella E, Nocetti L, Currà S, Ligabue G, Costi T, Torricelli P, Facchinetti F. Transvaginal ultrasound assessment of uterine scar after previous caesarean section: comparison with 3T-magnetic resonance diffusion tensor imaging. Radiol Med 2015;120:228-38. [Crossref] [PubMed]
- Souza JP, Gülmezoglu A, Lumbiganon P, Laopaiboon M, Carroli G, Fawole B, Ruyan PWHO Global Survey on Maternal and Perinatal Health Research Group. Caesarean section without medical indications is associated with an increased risk of adverse short-term maternal outcomes: the 2004-2008 WHO Global Survey on Maternal and Perinatal Health. BMC Med 2010;8:71. [Crossref] [PubMed]
- Zou Z, Xiao S, Xue M. Clinical analysis of the preoperative condition and operative prognosis of post-cesarean section scar diverticulum: a case series. J Perinat Med 2020;48:803-10. [Crossref] [PubMed]
- Yao M, Wang W, Zhou J, Sun M, Zhu J, Chen P, Wang X. Cesarean section scar diverticulum evaluation by saline contrast-enhanced magnetic resonance imaging: The relationship between variable parameters and longer menstrual bleeding. J Obstet Gynaecol Res 2017;43:696-704. [Crossref] [PubMed]


