
Solitary gastric metastasis from invasive lobular breast cancer misdiagnosed as gastritis: a case description
Introduction
Breast cancer ranks first in incidence rate among all female cancers (1). Invasive lobular carcinoma (ILC) is the second most common type of invasive breast cancer, accounting for approximately 10% of all invasive breast cancers (2). Distant metastasis of breast cancer commonly occurs in the lungs, liver, and bone (3), but gastric metastasis is rare, accounting for approximately 0.3% of cases in retrospective series (4,5). Due to this rarity, only sporadic cases of gastric metastases from breast cancer have been reported. Limited information is available on the clinicopathological characteristics, imaging findings, and endoscopic features of patients with gastric metastases of breast cancer. When breast cancer metastasizes to the stomach, it is sometimes misdiagnosed as primary tumor of the stomach or as gastritis (6), eventually affecting the choice of treatment and prognosis. In this study, we report a case of gastric metastasis from invasive lobular breast cancer, which was misdiagnosed as gastritis.
Case presentation
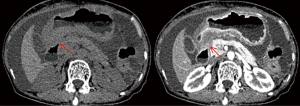
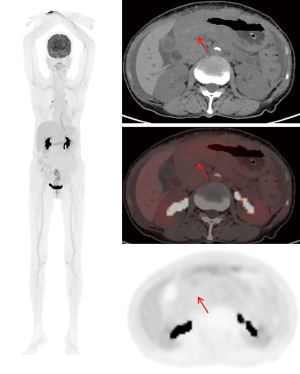
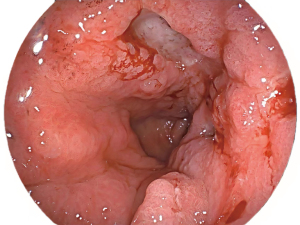
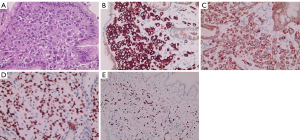
A 73-year-old woman with recurrent upper abdominal pain and indigestion for 6 months and vomiting for 3 days was admitted to our hospital. The patient was diagnosed with ILC of the breast at another hospital 1 year prior and did not undergo any treatment. For seeking further treatment, she consulted another hospital for the abovementioned digestive symptoms, and gastroscopy results suggested pyloric stenosis and chronic active gastritis. In our hospital, abdominal computed tomography (CT) enhancement showed that the wall of the gastric antrum was markedly thickened, with the thickest gastric wall being approximately 26 mm and causing localized narrowing of the gastric lumen. The lesion showed moderate enhancement during the arterial phase, which decreased during the venous phase, and further decreased during the delayed phase, with a net CT increase of approximately 32–47 Hounsfield units (HUs) (Figure 1). The fat interstitial space around the lesion was clear. The abovementioned manifestations suggested the presence of inflammatory lesions or tumor lesions. Fluorine 18 (18F) fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT (Figure 2) revealed slight FDG activity in the thickened gastric antrum [maximum standardized uptake value (SUVmax) 4.2], suggesting a higher likelihood of an inflammatory lesion than of a tumor. Based on these imaging findings, the patient underwent gastroscopy, which showed obvious swelling, hyperemia, and multiple ulcers in the mucosa of the gastric antrum (Figure 3). Pathological examinations showed infiltration of the gastric antrum wall by poorly differentiated adenocarcinoma cells (Figure 4A). Immunohistochemical analysis showed that the lesion was positive for cytokeratin (CK)7, CK-pan, GATA binding protein 3 (GATA3), and Ki-67 (~50%), and negative for CK20, mucin 6 (MUC6), CD163, human epidermal growth factor receptor 2 (HER2), and leukocyte common antigen (LCA) (Figure 4B-4E). These results indicated that the gastric sinus lesion was gastric metastasis of primary breast cancer. Surgery was not the preferred treatment choice, and the patient refused gastroduodenal stenting. Thus, palbociclib was used to treat the gastric metastases. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Gastric metastases from breast cancer are relatively rare. Furthermore, lobular breast cancer is reported to be more likely to involve gastric metastases than is invasive ductal carcinoma (7). Definitive diagnosis depends on postoperative pathology and immunohistochemistry. Thus, it is difficult to diagnose breast cancer with gastric metastases, and a high rate of misdiagnosis has been observed. Incorrect diagnosis seriously affects the prognosis and survival rate of patients.
Gastric metastasis usually occurs several years after the initial diagnosis of breast cancer with multiple metastases (8), and most gastric metastases occur simultaneously with systemic metastases (9). The clinical symptoms are insidious and nonspecific, often manifesting as upper abdominal pain, indigestion, vomiting, and nausea, among other symptoms. Given these symptoms, a primary gastric lesion and side effects of treatments such as chemotherapy cannot be ruled out. Solitary gastric metastasis in this case occurred 1 year after the diagnosis of breast cancer and manifested as recurrent episodes of epigastric pain and dyspepsia. No clear evidence of metastasis to other organs was found on imaging. This occurrence is extremely rare, and the mechanism remains unclear. Further reports on similar new cases are needed to address this issue.
Imaging findings as part of the diagnostic workup have been overlooked in previous case reports. According to a comprehensive literature review on gastric metastasis from breast cancer published in 2023 by Da Cunha et al. (10), imaging findings were reported in only 23 of 210 patients, 19 of which were CT and four of which were PET/CT. Previous reports did not elaborate on imaging presentation. In this case, abdominal CT enhancement suggested that the wall of the gastric antrum was markedly thickened, the lesion showed moderate enhancement, and the most significant strengthening amplitude was observed during the arterial phase. Furthermore, 18F-FDG PET/CT showed slight FDG activity in the thickened area of the gastric antrum (SUVmax 4.2), and no obvious abnormal FDG uptake was observed in other organs beyond the gastric region. Interestingly, of the four gastric cases that reported the use of PET scanning, two cases did not show increased FDG uptake (11,12) while the other two showed increased FDG activity at the exact location of the metastatic lesion (13,14). In this case, lower FDG uptake was observed at the lesion location as compared with that in previously reported cases. Thus, further information is needed regarding the sensitivity of FDG for detecting metastases in the gastrointestinal tract.
Normally, it is difficult to differentiate gastric metastases from primary gastric lesions on the basis of endoscopic findings alone. Endoscopic findings may be normal in approximately 50% of cases (15) because gastric metastases are mostly confined to the submucosal and muscular layers. In this case, even after several rounds of endoscopy and biopsy, the patient was not correctly diagnosed, probably since no biopsy was performed for the deeper gastric layers, including the submucosa.
Immunohistochemistry is considered the gold standard for differentiating gastric metastases (16). Gastric metastasis from invasive lobular breast cancer usually shows the same hormonal receptor expression to that of the primary breast cancer (3). In our case, gastric metastasis from breast cancer was positive for CK7, CK-pan, GATA3, and Ki-67 (~50%) and negative for CK20, MUC6, CD163, HER2, and LCA. GATA3 is a zinc-finger transcription factor of the GATA family that plays an important role in promoting and directing cell proliferation, development, and differentiation in various tissues and cell types. In normal tissues, GATA3 is often expressed in the nuclei of the breast, skin, kidney, and uroepithelial cells. In tumor tissues, GATA3 is expressed in primary and metastatic tumors of the breast (80–90%) and has also been used to identify uroepithelial and prostate cancers. CK20 and CK7 are members of the CK family, and CK7 is mostly negative in digestive tract tumors and positive in breast cancers; in contrast, CK20 is highly positive in all digestive tract tumors and negative in breast cancers (8). GATA3 and CK7 positivity and CK20 negativity in our patient suggest that the gastric antrum lesion originated from the breast.
In conclusion, we report a case of solitary gastric metastasis of ILC from the breast that was misdiagnosed as gastritis. According to our findings, when patients with breast cancer present with gastrointestinal symptoms (such as upper abdominal pain, indigestion, and vomiting) or gastric findings are detected by imaging, clinicians should be highly alert and should consider the presence of gastric metastasis from breast cancer and perform immunohistochemical analysis to achieve accurate diagnosis.
Acknowledgments
We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1461/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev 2017;26:444-57. [Crossref] [PubMed]
- McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and 'omics. Breast Cancer Res 2015;17:12. [Crossref] [PubMed]
- Rech MB, da-Cruz ER, Salgado K, Balbinot RA, Balbinot SS, Soldera J. Metastatic gastric cancer from breast carcinoma presenting with paraneoplastic rheumatic syndrome: A case report. World J Clin Cases 2023;11:3282-7. [Crossref] [PubMed]
- Xu L, Liang S, Yan N, Zhang L, Gu H, Fei X, Xu Y, Zhang F. Metastatic gastric cancer from breast carcinoma: A report of 78 cases. Oncol Lett 2017;14:4069-77. [Crossref] [PubMed]
- Koike K, Kitahara K, Higaki M, Urata M, Yamazaki F, Noshiro H. Clinicopathological features of gastric metastasis from breast cancer in three cases. Breast Cancer 2014;21:629-34. [Crossref] [PubMed]
- Abid A, Moffa C, Monga DK. Breast cancer metastasis to the GI tract may mimic primary gastric cancer. J Clin Oncol 2013;31:e106-7. [Crossref] [PubMed]
- Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer 2000;89:2214-21.
- Kim DH, Son SM, Choi YJ. Gastric metastasis from invasive lobular breast cancer, mimicking primary gastric cancer: A case report. Medicine (Baltimore) 2018;97:e0258. [Crossref] [PubMed]
- Kaneko Y, Koi Y, Kajitani K, Ohara M, Daimaru Y. Asymptomatic solitary metastasis to the stomach from breast cancer: A case report. Mol Clin Oncol 2020;13:75. [Crossref] [PubMed]
- Da Cunha T, Restrepo D, Abi-Saleh S, Dharan M. Breast cancer metastasizing to the upper gastrointestinal tract (the esophagus and the stomach): A comprehensive review of the literature. World J Gastrointest Oncol 2023;15:1332-41. [Crossref] [PubMed]
- Whitty LA, Crawford DL, Woodland JH, Patel JC, Nattier B, Thomas CR Jr. Metastatic breast cancer presenting as linitis plastica of the stomach. Gastric Cancer 2005;8:193-7. [Crossref] [PubMed]
- Hara F, Kiyoto S, Takabatake D, Takashima S, Aogi K, Ohsumi S, Teramoto N, Nishimura R, Takashima S. Metastatic Breast Cancer to the Stomach Resembling Early Gastric Cancer. Case Rep Oncol 2010;3:142-7. [Crossref] [PubMed]
- Ricciuti B, Leonardi GC, Ravaioli N, De Giglio A, Brambilla M, Prosperi E, Ribacchi F, Meacci M, Crinò L, Maiettini D, Chiari R, Metro G. Ductal Breast Carcinoma Metastatic to the Stomach Resembling Primary Linitis Plastica in a Male Patient. J Breast Cancer 2016;19:324-9. [Crossref] [PubMed]
- Geredeli C, Dogru O, Omeroglu E, Yilmaz F, Cicekci F. Gastric Metastasis of Triple Negative Invasive Lobular Carcinoma. Rare Tumors 2015;7:5764. [Crossref] [PubMed]
- Qu Q, Zong Y, Fei XC, Chen XS, Xu C, Lou GY, Shen KW. The importance of biopsy in clinically diagnosed metastatic lesions in patients with breast cancer. World J Surg Oncol 2014;12:93. [Crossref] [PubMed]
- Yang W, Ding S, Wang L, Ren F, Lai Y, Wang H, Wang H, Hong G, Gao W. Carcinoma with signet ring cell differentiation associated with invasive breast cancer: A case report. Oncol Lett 2023;25:212. [Crossref] [PubMed]


