
Potential diverse applications of diffusion-derived vessel density (DDVD) pixel-by-pixel mapping
On diffusion-weighted (DW) imaging, blood vessels show high signal when there is no diffusion gradient (b=0 s/mm2), while they show low signal even when very low b-values (such as b=1 or 2 s/mm2) are applied. Thus, the signal difference between images when the diffusion gradient is off and images when the diffusion gradient is on reflects the extent of tissue vessel density. Recently, Wáng (1) proposed that liver tissue micro-perfusion can be measured by a DW imaging-derived surrogate biomarker [diffusion-derived vessel density (DDVD)]:
where ROIarea0 and ROIarea2 refer to the number of pixel in the selected region-of-interest (ROI) on b=0 s/mm2 and b=2 s/mm2 images, respectively. Sb0 refers to the measured total liver signal intensity within the ROI when b=0 s/mm2, and Sb2 refers to the measured total liver signal intensity within the ROI when b=2 s/mm2, thus Sb/ROIarea equates to the mean signal intensity within the ROI. Sb2 and ROIarea2 can also be approximated by other low b-value diffusion image’s data. DDVD can be interpreted as a physiological surrogate of the area of micro-vessels per unit tissue area, which can be conceptually converted to a surrogate of the volume of micro-vessels per tissue unit volume if multiple slices are integrated.
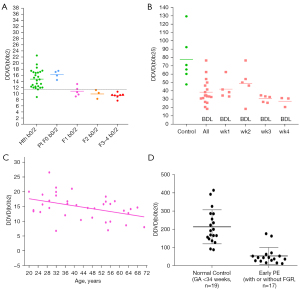
The potential of clinical application of DDVD as a straightforward diffusion imaging biomarker has been recently demonstrated (1-6) (Figure 1). DDVD was initially proposed to evaluate the perfusion status of liver fibrosis, and it has shown that it is a useful parameter for the distinguishing of livers with and without fibrosis, and that livers with severer fibrosis tend to have even lower DDVD measurements than those with milder liver fibrosis (1-3). DDVD has also been tested in a number of other clinical scenarios (4-7). Zheng et al. (4) described spleen DDVD is decreased in viral hepatitis-b liver fibrosis patients. Huang et al. (5) showed that DDVD analysis demonstrates liver parenchyma has an age-dependent decrease of micro-perfusion. This agrees with the known physiological age-dependent reduction in liver blood flow which has been well documented using a variety of technical methods including histology, dye dilution, and indicator clearance. He et al. (6) reported that DDVD analysis of the placenta allowed excellent separation of normal and early preeclampsia pregnancies. In a pilot study of 72 hepatocellular carcinoma (HCC) patients, the median ratio of tumor DDVD to adjacent liver DDVD was 2.94, which agrees with contrast agent dynamically enhanced computed tomography/magnetic resonance imaging (CT/MRI) data (7). Mean DDVD value was higher for HCCs with micro-vessel invasion (MVI) than HCCs without MVI, and mean DDVD value was higher for more malignment Edmondson-Steiner grade III or IV HCCs than for better differentiated Edmondson-Steiner grade I or II HCCs (7).
Compared with existing perfusion imaging techniques, DDVD protocol has many advantages. Compared with contrast agent dynamically enhanced imaging, DDVD protocol does not involve contrast injection, data acquisition is much faster, and data post-processing is also relatively straightforward. Compared with contrast agent enhanced CT perfusion examination, DDVD is without radiation. Compared with contrast agent enhanced MRI perfusion examination, our initial experience suggests that DDVD measure is more stable. The analysis of DDVD requires only 2 b-values (with one being b=0 s/mm2), allowing a short scanning time that can be completed within a single breath-hold duration, rendering it useful when the target organ is subject to respiratory motion. Intravoxel incoherent motion (IVIM) is also a noninvasive imaging method (but with a long scan time). However, it has been shown when there is T2 relaxation elevation of tumor (or other pathological tissue) relative to native tissue, standard IVIM modeling leads to suppression of perfusion fraction (and maybe also Dfast) measurement (8,9). The spleen is known to have similar total blood perfusion per unit volume to that of the liver. However, due to the longer T2 of the spleen relative to the T2 of the liver, IVIM analysis showed that spleen perfusion fraction is only half of that of the liver (10). On the other hand, T2 shortening leads to artificial elevation of perfusion fraction and Dfast measurement (11). Jerome et al. proposed a T2 extend IVIM model to correct this phenomenon, but this approach requires IVIM data acquisition to be run multiple times with varying TEs (12,13). This would lead to excessively long data acquisition in clinical settings.
As absolute magnetic resonance (MR) signal intensity is influenced by various factors, including B0/B1 spatial inhomogeneity, coil loading, receiver gain, etc., we have used the ratio of a lesion to its adjacent native tissue (such as the ratio of HCC’s DDVD to liver DDVD) to minimize these scaling factors (7). Following this, it may be useful to present pixel-by-pixel map of DDVD, so that the difference between the DDVD of a diseased tissue and DDVD of native/adjacent tissues can be compared visually. In this article, we present some examples of such DDVD map (Figures 2-12). We try to convey the message that DDVD map can be applied in multiple organs and multiple tissues, helping to evaluate different pathological entities particularly concerning their perfusion status. It has been shown apparent diffusion coefficient (ADC) measure is heavily affected by T2 effect (14,15); however, T2 effect can be minimized for DDVD when appropriate scan protocols or data processing protocols are adopted (Figure 5).
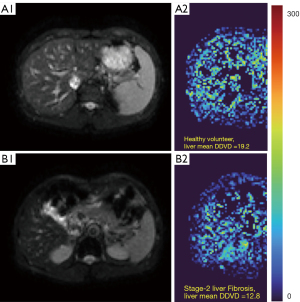
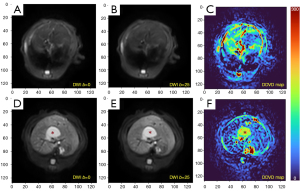
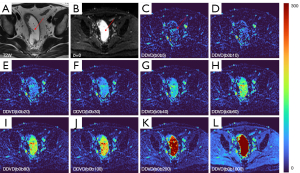
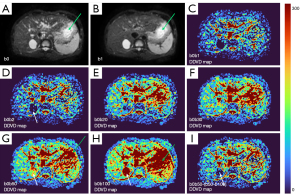
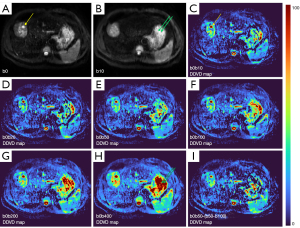
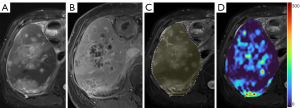
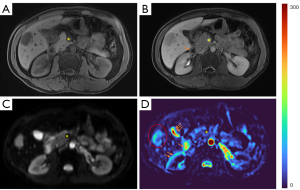

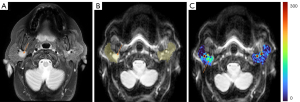
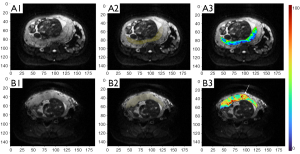
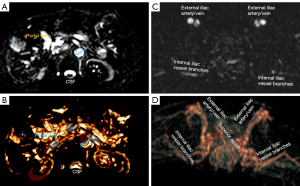
How to acquire DDVD scan protocol and how to conduct image postprocessing have not been optimized yet. Increasing number of excitations (NEX) can improve DDVD measure stability, and since the DDVD protocol is very fast, high NEX is feasible. Figures 2-11 show that DDVD map should be viewed side-by-side with the source DW images. The clinical application potential of DDVD map will be further studied.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Quantitative Imaging in Medicine and Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-164/coif). Y.X.J.W. serves as the Editor-in-Chief of Quantitative Imaging in Medicine and Surgery. Y.X.J.W. is the founder of Yingran Medicals Ltd., which develops medical image-based diagnostics software. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wáng YXJ. Living tissue intravoxel incoherent motion (IVIM) diffusion MR analysis without b=0 image: an example for liver fibrosis evaluation. Quant Imaging Med Surg 2019;9:127-33. [Crossref] [PubMed]
- Xiao BH, Huang H, Wang LF, Qiu SW, Guo SW, Wáng YXJ. Diffusion MRI Derived per Area Vessel Density as a Surrogate Biomarker for Detecting Viral Hepatitis B-Induced Liver Fibrosis: A Proof-of-Concept Study. SLAS Technol 2020;25:474-83. [Crossref] [PubMed]
- Hu GW, Zheng CJ, Zhong WX, Zhuang DP, Xiao BH, Wáng YXJ. Usefulness of diffusion derived vessel density computed from a simplified IVIM imaging protocol: An experimental study with rat biliary duct blockage induced liver fibrosis. Magn Reson Imaging 2021;84:115-23. [Crossref] [PubMed]
- Zheng CJ, Huang H, Xiao BH, Li T, Wang W, Wáng YXJ. Spleen in viral Hepatitis-B liver fibrosis patients may have a reduced level of per unit micro-circulation: non-invasive diffusion MRI evidence with a surrogate marker. SLAS Technol 2022;27:187-94. [Crossref] [PubMed]
- Huang H, Zheng CJ, Wang LF, Che-Nordin N, Wáng YXJ. Age and gender dependence of liver diffusion parameters and the possibility that intravoxel incoherent motion modeling of the perfusion component is constrained by the diffusion component. NMR Biomed 2021;34:e4449. [Crossref] [PubMed]
- He J, Chen C, Xu L, Xiao B, Chen Z, Wen T, Wáng YXJ, Liu P. Diffusion-Derived Vessel Density Computed From a Simplified Intravoxel Incoherent Motion Imaging Protocol in Pregnancies Complicated by Early Preeclampsia: A Novel Biomarker of Placental Dysfunction. Hypertension 2023;80:1658-67. [Crossref] [PubMed]
- Li XM, Yao DQ, Quan XY, Li M, Chen W, Wáng YXJ. Perfusion of hepatocellular carcinomas (HCC) measured by diffusion-derived vessel density (DDVD) biomarker: higher HCC perfusion than earlier IVIM reports. NMR Biomed 2024; [Crossref]
- Ma FZ, Wáng YXJ T. (2) relaxation time elongation of hepatocellular carcinoma relative to native liver tissue leads to an underestimation of perfusion fraction measured by standard intravoxel incoherent motion magnetic resonance imaging. Quant Imaging Med Surg 2024;14:1316-22. [Crossref] [PubMed]
- Wáng YXJ, Sabarudin A. Underestimation of liver hemangioma perfusion fraction by standard intravoxel incoherent motion diffusion magnetic resonance imaging. Quant Imaging Med Surg 2024;14:2128-35. [Crossref] [PubMed]
- Yu WL, Xiao BH, Ma FZ, Zheng CJ, Tang SN, Wáng YXJ. Underestimation of the spleen perfusion fraction by intravoxel incoherent motion MRI. NMR Biomed 2023;36:e4987. [Crossref] [PubMed]
- Xiao BH, Wáng YXJ. Different tissue types display different signal intensities on b = 0 images and the implications of this for intravoxel incoherent motion analysis: Examples from liver MRI. NMR Biomed 2021;34:e4522. [Crossref] [PubMed]
- Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 2010;64:1580-5. [Crossref] [PubMed]
- Jerome NP, d'Arcy JA, Feiweier T, Koh DM, Leach MO, Collins DJ, Orton MR. Extended T2-IVIM model for correction of TE dependence of pseudo-diffusion volume fraction in clinical diffusion-weighted magnetic resonance imaging. Phys Med Biol 2016;61:N667-80.
- Wáng YXJ, Zhao KX, Ma FZ, Xiao BH. The contribution of T2 relaxation time to MRI-derived apparent diffusion coefficient (ADC) quantification and its potential clinical implications. Quant Imaging Med Surg 2023;13:7410-6. [Crossref] [PubMed]
- Wáng YXJ, Ma FZ. A tri-phasic relationship between T2 relaxation time and magnetic resonance imaging (MRI)-derived apparent diffusion coefficient (ADC). Quant Imaging Med Surg 2023;13:8873-80. [Crossref] [PubMed]


