
Developing and validating a prediction model of live birth in patients with moderate-to-severe intrauterine adhesions: a new approach with endometrial morphology measurement by 3D transvaginal ultrasound
Introduction
Intrauterine adhesions (IUA) are intrauterine or cervical adhesions caused by damage of the endometrial basal layer. They are usually accompanied by endometrial fibrosis, and mainly manifested by reduced menstrual volume, amenorrhea, and periodic lower abdominal pain, which has a significant impact on female fertility and can lead to recurrent miscarriage or infertility (1). Induced abortion or curettage significantly increases the incidence of IUA (2). IUA may cause pregnancy complications, such as spontaneous abortion, premature delivery, and placenta implantation, which increases the probability of uterine curettage and further compromises the fertility of patients (3,4). Therefore, we believe that the pregnancy outcome of patients with IUA should be paid more attention. Live birth is a valuable indicator of pregnancy outcome and the main appeal for patients with IUA with fertility needs. Therefore, in the clinical work, how to comprehensively evaluate the postoperative live birth rate of patients with IUA and provide more practical suggestions for patients is worthy of further discussion.
Some researchers have investigated the factors influencing the postoperative live birth of patients with IUA. Cao et al. (5) studied the correlation between different IUA evaluation systems following hysteroscopic adhesiolysis (HA) and live birth after surgery in 128 patients with IUA and found that Nasr classification (Nasr) had the highest predictive value for live birth. Zhang et al. (6) studied the high-risk factors of obstetric complications in 265 patients with IUA and found that the degree of intrauterine involvement and the number of surgical abortions were risk factors for placenta accreta spectrum. In another study, the clinical characteristics of 394 patients with moderate-to-severe IUA were analyzed retrospectively by constructing a decision tree model. The menstrual pattern after HA was found to be the key factor in predicting live birth (7). Another study, which included 71 patients who underwent HA, identified age as a key factor in assessing fertility after surgery (8).
We have discussed the predictive ability of live birth after HA from the aspects of pregnancy patterns, uterine cavity parameters, preoperative 3D transvaginal ultrasound (3D-TVUS) parameters, and endometrial gland density (9-12). Assisted reproductive technology (ART) might be a better choice for patients with recurrent IUA (9). Visible bilateral fallopian tube ostia or American Fertility Society (AFS) scores of <4 in the hysteroscopic reexamination might predict better live birth (10). Thin endometrium and the upper and middle segments of endometrial absence have been found to be risk factors for live birth (11). We also found that the density of endogenous glandular openings can be a novel variable to predict live births in patients with IUA (12).
A 3D-TVUS examination is commonly completed before hysteroscopy to evaluate the disease, but not enough attention has been paid to the value of 3D-TVUS examination after HA. Furthermore, the ability of the post-HA 3D-TVUS to predict the live birth rate of patients with IUA is still unclear. Moreover, most of the previous articles used univariate and multivariate logistic regression analysis to evaluate the risk factors affecting the likelihood of postoperative live birth for patients with IUA; they did not establish and validate a clinical prognosis. Finally, this study has a larger sample size (870 patients) than that in our previous research, which can help to build a more practical prognosis model. A nomogram using the results of 3D-TVUS after second-look hysteroscopy was built based on 870 patients with moderate-to-severe IUA to predict the live birth rate. We present this article in accordance with the TRIPOD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1014/rc).
Methods
Patients
A total of 870 patients with fertility requirements were retrospectively enrolled in this study. From January 2018 to February 2020, all participants underwent HA in the Department of Gynecology, Third Xiangya Hospital, Central South University. The included patients were selected through the same method.
All cases were followed up for 2 years by the same person, and the pregnancy outcome was recorded. The patients were followed up every 3 months from 1 year after second-look hysteroscopy. The pregnant patients were followed up until the termination of the pregnancy. Patients without pregnancy were followed up to 2 years after operation. For patients with multiple pregnancies, only the first pregnancy outcome was recorded. The time of the end of follow-up was April 2022.
The inclusion criteria of this study were as follows: (I) patients with reproductive needs; (II) patients who underwent HA at the Third Xiangya Hospital of Central South University; (III) patients with IUA diagnosed by hysteroscopy; (IV) patients with moderate-to-severe IUA; and (V) availability of 3D-TVUS results during the interval between second-look hysteroscopy and pregnancy. The exclusion criteria were as follows: (I) endometrial tuberculosis; (II) severe adhesion rendered the restoration of the normal uterine cavity impossible; (III) infertility with other causes, such as male infirmity, tubal factor infertility; (IV) primary infertility; (V) uterine cavity volume either too large or too small, inability to place an intrauterine device (IUD) after surgery; or (VI) loss to follow-up.
Clinical data collection
We obtained each patient’s demographic information (age), clinical history (disease course, pregnancy patterns, pregnancy outcomes, gravidity, parity, spontaneous abortion, surgical abortion/curettage, and recurrence of IUA), 3D-TVUS results after second-look hysteroscopy (uterine length, endometrial thickness, distance between two uterine cornua, triline sign, endometrial echo, uterine mobility, blood flow of endometrium, and endometrial mobility), results of the second-look hysteroscopy (segmentation of scar contraction, segmentation of the endometrial absence, IUA score, and the number of visible tubal ostia) from the electronic medical record. The medical records, operative reports, and hysteroscopy videos of all patients were reviewed by two doctors. The main outcome indicators were pregnancy outcomes, including live birth and non-live birth. Non-live birth was defined as infertility, induced abortion, spontaneous abortion, induced labor, and stillbirth. Disease course referred to the time interval between the occurrence of reduced menstrual volume, amenorrhea, or IUA detected by B-ultrasound examination or hysteroscopy. The IUA scores, also known as the AFS score, were categorized as follows (13): 1–4 (mild), 5–8 (moderate), and 9–12 (severe).
Surgical procedure for the last HA
Second-look hysteroscopy was conducted 1 month after the initial HA for patients with severe IUA (score 9–12) and 3 months after the initial HA for patients with moderate IUA (score 5–8) (12).
HA was performed within 3–7 days following menstruation. An operative hysteroscope (Karl Storz SE & Co. KG. KG-Tuttlingen, Baden-Württemberg, Germany) was used. A blunt spreading dissection technique (14) and the cold scissors ploughing technique (15) were used to dissect adhesive tissues and reconstruct the normal uterine cavity. An IUD was placed during the first HA and removed during the second-look hysteroscopy.
For patients who wished to become pregnant, bilateral intubation of the fallopian tubes under hysteroscopy was completed (9). After the HA, 3 mL of hyaluronic acid gel was then injected into the uterine cavity via the catheter (16).
3D-TVUS examination
In this study, 3D-TVUS was performed at the middle stage of the endometrial secretory phase after the second-look hysteroscopy and before pregnancy using a GE volume E8 ultrasonic instrument (GE Healthcare GmbH & Co OG, Tiefenbach, Styria, Austria). The 3D-TVUS examination was conducted by ultrasound doctors with more than 10 years of clinical experience during the period from the last HA to the pregnancy.
The endometrium was divided into patterns A, B, and C, which were distinguished by two hyperechoic lines between the endometrium and the myometrium and by different images of a hyperechoic central line between the two layers of the endometrium (17). Pattern A meant that three lines of endometrium can be clearly displayed. Pattern B referred to an endometrial echo that is uniform and moderate, and the strong echo line of the uterus is discontinuous or unclear. In pattern C, the endometrium showed a strong homogeneous echo, no midline echo in the uterine cavity, and three lines disappeared (17).
Endometrial blood flow was divided into grades 0, 1, 2, and 3 (18). Grade 0 means that the blood flow signal can hardly be detected in the endometrium. Grade 1 refers to the blood flow reaching the basal layer of the endometrium. Grade 2 refers to the blood flow reaching the functional layer of the endometrium. Grade 3 refers to the blood flow reaching the central lining of the urinary cavity (18). We combined grades 2 and 3 patients into the grade ≥2 group. Endometrial wave-like movements were classified according to the classification system constructed by Ijland et al. (19): no activity, no activity detected during at least 2 minutes when recording; fundo-cervical, waves moving from the fundus to the cervix; cervico-fundal, waves moving from the cervix to the fundus; opposing, waves produced from both cervix and fundus; and random, waves generated at any point on the uterus. In this study, bidirection included fundo-cervical and cervico-fundal; irregular included opposing and random.
Analysis plan
The statistical software SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) were used to analyze the data and draw graphs. The normality test was carried out by Shapiro-Wilk method. The count data were expressed by frequency percentage (%), and the comparison between groups was performed using a Z test. Continuous variables were compared by the independent-samples t-test, or the Mann-Whitney U test. The predictors were screened by univariate and multivariate logistic regression analysis, and the odds ratio (OR) and 95% confidence interval (CI) were calculated. The predictive factors were included in the clinical predictive model, and a nomogram was constructed. The model was evaluated by the receiver operating characteristic (ROC) curve, calibration curve, the Hosmer-Lemeshow (HL) test statistic, and decision curve analysis (DCA). Spearman correlation analysis was used to analyze the correlation between clinical parameters and ultrasound parameters, which was shown by a heat map. A P value <0.05 was considered statistically significant.
Ethical statement
This study was approved by the Ethics Committee of Third Xiangya Hospital of Central South University (IRB No. I 21053). All participants signed an informed consent form. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and all the terms related to human study participants.
Results
According to the inclusion criteria, 922 patients with IUA were initially included in this study, and 52 patients were excluded according to the following criteria: (I) male competence (n=8); (II) tubercular IUA (n=1); (III) tubal factor infertility (n=6); (IV) other lesions, including endometrial polyps, atypical hyperplasia, or endometrial malignancy (n=14); and (V) lost to follow-up (n=23). Finally, 870 patients were included in the final study and divided into groups according to the ratio of 7:3, including 609 in the derivation cohort and 261 in the validation cohort (Figure 1). Table 1 shows the clinical characteristics of 870 patients with IUA with complete follow-up data on their pregnancy outcomes. In the two groups, the average age of the patients was 31.0 years old (28.0–35.0 years), and all patients were of reproductive age. A total of 84.71% of patients were hoping to achieve a spontaneous pregnancy after HA, and only 15.29% chose ART. There were 558 live births, about twice the number of non-live births. Most cases had non-recurrent IUA (64.70% vs. 35.30%). The constituent ratio of moderate and severe patients with IUA was similar between the two groups (48.74% vs. 51.26%). There was no significant difference in all clinical parameters between the derivation and validation cohorts (P>0.05).
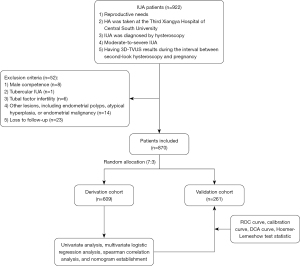
Table 1
| Variable | Total (n=870) | Derivation (n=609) | Validation (n=261) | Z/χ2 | P value |
|---|---|---|---|---|---|
| Age (year) | 31.0 (28.0–35.0) | 32.0 (28.0–36.0) | 31.0 (28.0–34.0) | 1.377 | 0.168 |
| Disease course (year) | 0.8 (0.3–2.0) | 0.7 (0.3–2.0) | 0.8 (0.3–2.0) | 0.824 | 0.410 |
| Pregnancy patterns | 0.711 | 0.399 | |||
| Spontaneous pregnancy | 737 (84.71) | 520 (85.39) | 217 (83.14) | ||
| ART | 133 (15.29) | 89 (14.61) | 44 (16.86) | ||
| Pregnancy outcomes | 0.161 | 0.688 | |||
| Non-live birth | 312 (35.86) | 221 (36.29) | 91 (34.87) | ||
| Live birth | 558 (64.14) | 388 (63.71) | 170 (65.13) | ||
| Gravidity | 2.180 | 0.536 | |||
| 0 | 25 (2.87) | 19 (3.12) | 6 (2.30) | ||
| 1 | 162 (18.62) | 111 (18.23) | 51 (19.54) | ||
| 2 | 247 (28.39) | 166 (27.26) | 81 (31.03) | ||
| ≥3 | 436 (50.11) | 313 (51.40) | 123 (47.13) | ||
| Parity | 2.333 | 0.311 | |||
| 0 | 523 (60.11) | 356 (58.46) | 167 (63.98) | ||
| 1 | 302 (34.71) | 220 (36.12) | 82 (31.42) | ||
| ≥2 | 45 (5.17) | 33 (5.42) | 12 (4.60) | ||
| Spontaneous abortion | 2.062 | 0.560 | |||
| 0 | 41 (4.71) | 31 (5.09) | 10 (3.83) | ||
| 1 | 261 (30.00) | 176 (28.90) | 85 (32.57) | ||
| 2 | 244 (28.05) | 169 (27.75) | 75 (28.74) | ||
| ≥3 | 324 (37.24) | 233 (38.26) | 91 (34.87) | ||
| Surgical abortion/curettage | 0.112 | 0.990 | |||
| 0 | 45 (5.17) | 32 (5.25) | 13 (4.98) | ||
| 1 | 412 (47.36) | 287 (47.13) | 125 (47.89) | ||
| 2 | 195 (22.41) | 138 (22.66) | 57 (21.84) | ||
| ≥3 | 218 (25.06) | 152 (24.96) | 66 (25.29) | ||
| Previous HA history | 2.071 | 0.150 | |||
| No | 576 (66.21) | 394 (64.70) | 182 (69.73) | ||
| Yes | 294 (33.79) | 215 (35.30) | 79 (30.27) | ||
| Uterine length (mm) | 28.0 (24.5–31.5) | 28.0 (25.0–31.5) | 28.0 (24.0–30.5) | 1.336 | 0.182 |
| Endometrial thickness (mm) | 5.0 (4.0–6.2) | 5.0 (4.0–6.2) | 5.0 (3.9–6.1) | 0.499 | 0.618 |
| Intercornual distance (mm) | 0.071 | 0.965 | |||
| ≤22 | 347 (39.89) | 242 (39.74) | 105 (40.23) | ||
| 23–25 | 224 (25.75) | 156 (25.62) | 68 (26.05) | ||
| ≥26 | 299 (34.37) | 211 (34.65) | 88 (33.72) | ||
| Triline sign | 3.896 | 0.273 | |||
| Not clear | 782 (89.89) | 548 (89.98) | 234 (89.66) | ||
| Pattern A | 17 (1.95) | 9 (1.48) | 8 (3.07) | ||
| Pattern B | 34 (3.91) | 27 (4.43) | 7 (2.68) | ||
| Pattern C | 37 (4.25) | 25 (4.11) | 12 (4.60) | ||
| Endometrial echo | 1.657 | 0.198 | |||
| Heterogeneous | 548 (62.99) | 392 (64.37) | 156 (59.77) | ||
| Homogeneous | 322 (37.01) | 217 (35.63) | 105 (40.23) | ||
| Uterine mobility | 0.480 | 0.787 | |||
| Poor | 35 (4.02) | 25 (4.11) | 10 (3.83) | ||
| Not good | 643 (73.91) | 446 (73.23) | 197 (75.48) | ||
| Well | 192 (22.07) | 138 (22.66) | 54 (20.69) | ||
| Blood flow of endometrium | 1.195 | 0.550 | |||
| Grade 0 | 227 (26.09) | 156 (25.62) | 71 (27.20) | ||
| Grade 1 | 592 (68.05) | 414 (67.98) | 178 (68.20) | ||
| Grade ≥2 | 51 (5.86) | 39 (6.40) | 12 (4.60) | ||
| Endometrial mobility | 1.233 | 0.540 | |||
| No | 531 (61.03) | 379 (62.23) | 152 (58.24) | ||
| Irregular | 290 (33.33) | 197 (32.35) | 93 (35.63) | ||
| Bidirection | 49 (5.63) | 33 (5.42) | 16 (6.13) | ||
| Segmentation of scar contraction | 3.336 | 0.343 | |||
| No | 341 (39.20) | 238 (39.08) | 103 (39.46) | ||
| Lower segment | 357 (41.03) | 259 (42.53) | 98 (37.55) | ||
| Middle segment | 125 (14.37) | 80 (13.14) | 45 (17.24) | ||
| Upper segment | 47 (5.40) | 32 (5.25) | 15 (5.75) | ||
| Segmentation of the endometrial absence | 3.588 | 0.310 | |||
| No | 267 (30.69) | 197 (32.35) | 70 (26.82) | ||
| Lower segment | 57 (6.55) | 37 (6.08) | 20 (7.66) | ||
| Middle segment | 306 (35.17) | 206 (33.83) | 100 (38.31) | ||
| Upper segment | 240 (27.59) | 169 (27.75) | 71 (27.20) | ||
| Number of visible tubal ostia | 1.273 | 0.529 | |||
| 0 | 170 (19.54) | 124 (20.36) | 46 (17.62) | ||
| 1 | 162 (18.62) | 109 (17.90) | 53 (20.31) | ||
| 2 | 538 (61.84) | 376 (61.74) | 162 (62.07) | ||
| AFS scores | 0.316 | 0.574 | |||
| 5–8 | 424 (48.74) | 293 (48.11) | 131 (50.19) | ||
| 9–12 | 446 (51.26) | 316 (51.89) | 130 (49.81) | ||
Continuous data are presented as the median (interquartile range) and categorical data are presented as number (%). ART, assisted reproductive technology; HA, hysteroscopic adhesiolysis; AFS, American Fertility Society.
In the derivation cohort, there were significant differences in some clinical indicators between the live birth and non-live birth groups. Patients with non-live births had the following characteristics: (I) older (median age, 32.0 vs. 30.0 years; P<0.001); (II) more patients chose spontaneous pregnancy (70.77% vs. 29.23%; P<0.001); and (III) the proportion of patients with pregnancy ≥3 times (67.73% vs. 32.27%; P=0.005) and delivery ≥2 times (72.73% vs. 27.27%; P<0.001) were higher. In the non-live birth group, more patients had experienced abortion, surgical abortion/curettage, and recurrence of IUA, but there was no significant difference between the two groups (P>0.05). For 3D-TVUS indicators, the endometrium was thicker (5.6 vs. 4.8 mm; P<0.001), and the intercornual distance was larger (P<0.001) in the live birth group, but patients in the non-live birth group were more likely to be heterogeneous endometrial echo (70.92% vs. 29.08%; P<0.001), poor uterine mobility (92.00% vs. 8.00%; P=0.01), poor endometrial blood flow (P<0.001), and worse endometrial mobility (P<0.001). For the variates in the second-look hysteroscopy, more patients in the non-live birth group had the following characteristics: (I) IUA in the middle segment of the uterus (80.00% vs. 20.00%; P<0.001) and the upper segment of the uterus (84.38% vs. 15.63%; P<0.001); (II) the missing part of the endometrium was located in the upper segment of the uterus (89.94% vs. 10.06%; P<0.001); (III) bilateral fallopian tube ostia could not be seen (82.26% vs. 17.74%; P<0.001); and (IV) more patients had severe IUA (71.84% vs. 28.16%; P<0.001; Table 2).
Table 2
| Variates | Non-live birth (n=388) | Live birth (n=211) | Z/χ2 | P value |
|---|---|---|---|---|
| Age (year) | 32.0 (29.0–37.0) | 30.0 (28.0–34.0) | 4.536 | <0.001 |
| Disease course (year) | 0.8 (0.3–2.0) | 0.6 (0.2–2.0) | 0.403 | 0.687 |
| Pregnancy patterns | 76.671 | <0.001 | ||
| Spontaneous pregnancy | 368 (70.77) | 152 (29.23) | ||
| ART | 20 (22.47) | 69 (77.53) | ||
| Gravidity | 12.872 | 0.005 | ||
| 0 | 7 (36.84) | 12 (63.16) | ||
| 1 | 60 (54.05) | 51 (45.95) | ||
| 2 | 109 (65.66) | 57 (34.34) | ||
| ≥3 | 212 (67.73) | 101 (32.27) | ||
| Parity | 18.014 | <0.001 | ||
| 0 | 202 (56.74) | 154 (43.26) | ||
| 1 | 162 (73.64) | 58 (26.36) | ||
| ≥2 | 24 (72.73) | 9 (27.27) | ||
| Spontaneous abortion | 3.967 | 0.265 | ||
| 0 | 16 (51.61) | 15 (48.39) | ||
| 1 | 106 (60.23) | 70 (39.77) | ||
| 2 | 111 (65.68) | 58 (34.32) | ||
| ≥3 | 155 (66.52) | 78 (33.48) | ||
| Surgical abortion/curettage | 1.882 | 0.597 | ||
| 0 | 17 (53.13) | 15 (46.88) | ||
| 1 | 184 (64.11) | 103 (35.89) | ||
| 2 | 87 (63.04) | 51 (36.96) | ||
| ≥3 | 100 (65.79) | 52 (34.21) | ||
| Recurrence of IUA | 2.001 | 0.157 | ||
| No | 243 (61.68) | 151 (38.32) | ||
| Yes | 145 (67.44) | 70 (32.56) | ||
| Uterine length (mm) | 28.0 (24.5–31.5) | 29.0 (25.0–32.0) | 1.168 | 0.243 |
| Endometrial thickness (mm) | 4.8 (3.7–5.8) | 5.6 (4.5–7.2) | 6.742 | <0.001 |
| Intercornual distance (mm) | 18.635 | <0.001 | ||
| ≤22 | 174 (71.90) | 68 (28.10) | ||
| 23–25 | 103 (66.03) | 53 (33.97) | ||
| ≥26 | 111 (52.61) | 100 (47.39) | ||
| Triline sign | 3.751 | 0.290 | ||
| Not clear | 355 (64.78) | 193 (35.22) | ||
| Pattern A | 6 (66.67) | 3 (33.33) | ||
| Pattern B | 15 (55.56) | 12 (44.44) | ||
| Pattern C | 12 (48.00) | 13 (52.00) | ||
| Endometrial echo | 24.718 | <0.001 | ||
| Heterogeneous | 278 (70.92) | 114 (29.08) | ||
| Homogeneous | 110 (50.69) | 107 (49.31) | ||
| Uterine mobility | 9.232 | 0.010 | ||
| Poor | 23 (92.00) | 2 (8.00) | ||
| Not good | 281 (63.00) | 165 (37.00) | ||
| Well | 84 (60.87) | 54 (39.13) | ||
| Blood flow of endometrium | 22.471 | <0.001 | ||
| Grade 0 | 115 (73.72) | 41 (26.28) | ||
| Grade 1 | 260 (62.80) | 154 (37.20) | ||
| Grade ≥2 | 13 (33.33) | 26 (66.67) | ||
| Endometrial mobility | 20.115 | <0.001 | ||
| No | 267 (70.45) | 112 (29.55) | ||
| Irregular | 102 (51.78) | 95 (48.22) | ||
| Bidirection | 19 (57.58) | 14 (42.42) | ||
| Segmentation of scar contraction | 18.946 | <0.001 | ||
| No | 146 (61.34) | 92 (38.66) | ||
| Lower segment | 151 (58.30) | 108 (41.70) | ||
| Middle segment | 64 (80.00) | 16 (20.00) | ||
| Upper segment | 27 (84.38) | 5 (15.63) | ||
| Segmentation of the endometrial absence | 73.280 | <0.001 | ||
| No | 112 (56.85) | 85 (43.15) | ||
| Lower segment | 15 (40.54) | 22 (59.46) | ||
| Middle segment | 109 (52.91) | 97 (47.09) | ||
| Upper segment | 152 (89.94) | 17 (10.06) | ||
| Number of visible tubal ostia | 28.006 | <0.001 | ||
| 0 | 102 (82.26) | 22 (17.74) | ||
| 1 | 74 (67.89) | 35 (32.11) | ||
| 2 | 212 (56.38) | 164 (43.62) | ||
| AFS scores | 18.751 | <0.001 | ||
| 5–8 | 161 (54.95) | 132 (45.05) | ||
| 9–12 | 227 (71.84) | 89 (28.16) | ||
Continuous data are presented as the median (interquartile range) and categorical data are presented as number (%). HA, hysteroscopic adhesiolysis; ART, assisted reproductive technology; IUA, intrauterine adhesions; AFS, American Fertility Society.
Multivariate logistic regression analysis showed that age (OR 0.929; 95% CI: 0.893–0.967; P<0.001), endometrial thickness (OR 1.276; 95% CI: 1.150–1.416; P<0.001), ART (OR 7.016; 95% CI: 3.856–12.766; P<0.001), homogeneous endometrial echo (OR 1.797; 95% CI: 1.171–2.759; P=0.007), lower segment of scar contraction (OR 2.259; 95% CI: 1.390–3.672; P=0.001), and upper segmentation of the endometrial absence (OR 0.159; 95% CI: 0.080–0.318; P<0.001) were independent predictors for pregnancy outcomes of patients with IUA after HA in the derivation cohort (Table 3).
Table 3
| Variable | Category | Estimate | SE | Wald | P value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Low | Up | |||||||
| Intercept | –0.142 | – | 0.035 | 0.851 | 0.867 | –0.615 | 2.349 | |
| Age | –0.073 | –0.213 | 12.801 | <0.001 | 0.929 | 0.893 | 0.967 | |
| Endometrial thickness | 0.244 | 0.264 | 21.080 | <0.001 | 1.276 | 1.150 | 1.416 | |
| Pregnancy patterns | ART | 1.948 | 0.380 | 40.691 | <0.001 | 7.016 | 3.856 | 12.766 |
| Endometrial echo | Homogeneous | 0.586 | 0.155 | 7.182 | 0.007 | 1.797 | 1.171 | 2.759 |
| Segmentation of scar contraction | Lower segment | 0.815 | 0.222 | 10.803 | 0.001 | 2.259 | 1.390 | 3.672 |
| Middle segment | –0.673 | –0.125 | 3.124 | 0.077 | 0.510 | 0.242 | 1.076 | |
| Upper segment | 0.148 | 0.018 | 0.069 | 0.793 | 1.160 | 0.384 | 3.502 | |
| Segmentation of the endometrial absence | Lower segment | 0.249 | 0.033 | 0.337 | 0.562 | 1.283 | 0.553 | 2.980 |
| Middle segment | 0.358 | 0.093 | 2.217 | 0.137 | 1.430 | 0.893 | 2.290 | |
| Upper segment | –1.838 | –0.454 | 27.007 | <0.001 | 0.159 | 0.080 | 0.318 | |
IUA, intrauterine adhesions; SE, standard error; OR, odds ratio; CI, confidence interval; ART, assisted reproductive technology.
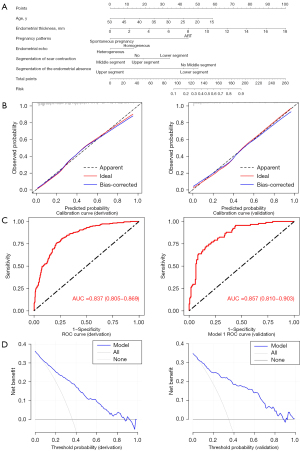
Next, we constructed a nomogram containing the six predictive factors (Figure 2A). The total score of the nomogram was the sum of the corresponding scores assigned to each risk factor. The higher the score, the higher the risk of live birth. In the HL test, there was no significant difference between the actual live birth rate and the predicted live birth rate (χ2=5.912; P=0.657), indicating that the goodness of fit of this model is high. In both cohorts, the blue line is a straight line with a slope close to 1, which indicates that there is a good correlation between the probability of actual live birth and the probability of predicted live birth (Figure 2B). According to ROC analysis, the area under the curve (AUC) was 0.837 (95% CI: 0.805–0.869) in the derivation cohort and 0.857 (95% CI: 0.810–0.903) in the validation cohort, which revealed good discrimination (Figure 2C). The DCA curve was used to evaluate the clinical practicability of the model. When the predictive rate of the model was about 0.35–0.90, there was a net benefit from clinical measures (Figure 2D).
Finally, to explore whether the 3D-TVUS results after the second-look hysteroscopy were consistent with the results during the second-look hysteroscopy, correlation analysis was used to study the correlation between the two kinds of parameters (Figure 3). The results showed that five of the 3D-TVUS results after the second-look hysteroscopy (endometrial thickness, distance between two uterine cornua, endometrial echo, blood flow of endometrium, and endometrial mobility) had a closer correlation with the results of the second-look hysteroscopy.
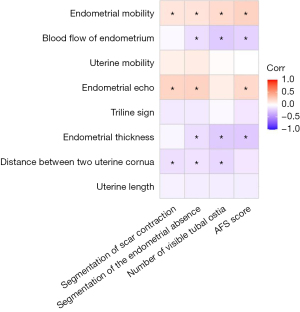
On the whole, endometrial echo or endometrial mobility had a positive correlation with the results in the second-look hysteroscopy (P<0.05). Endometrial thickness, the distance between two uterine cornua, or blood flow of endometrium had a negative correlation with the results in the second-look hysteroscopy (P<0.05). Homogeneous endometrial echo was related to the no segmentation of scar contraction (r=0.219; P<0.001) or no segmentation of the endometrial absence (r=0.226; P<0.001). Thicker endometrium was associated with no segmentation of the endometrial absence (r=−0.145; P=0.007). The results suggested that the outcomes of noninvasive 3D-TVUS and invasive hysteroscopy were consistent in their judgments of endometrial conditions.
Discussion
In this study, the predictive model suggested that endometrial thickness was an important factor affecting live birth. This finding is consistent with the results of other studies (20-22). However, it is often difficult to achieve a satisfactory endometrial thickness in patients with IUA (23), so the thin endometrium has become a difficult problem for doctors and patients (24). Endometrial thickness <7 mm is associated with lower pregnancy rates (24). The mean endometrial thickness of the 870 participants was only 5 mm (4.0–6.2 mm). The mean endometrial thickness was 5.6 mm (4.5–7.2 mm) in the live birth group, which was higher than the 4.8 mm (3.7–5.8 mm) in the non-live birth group, but lower than 7 mm. At present, sodium hyaluronate, estrogen, stem cell transplantation, amniotic membrane transplantation, and organoid transplantation have been reported to improve thin endometrium (25-28), but the effects are not significant. Therefore, how to improve the endometrial thickness of patients with IUA and improve the pregnancy outcome requires further investigation.
In addition, we should pay attention to the evaluation of endometrium by 3D-TVUS after HA and timely intervention. Usually, 3D-TVUS is performed before HA. However, during the period from the completion of HA to the time before pregnancy, 3D-TVUS has not attracted enough attention. After surgery, anatomical and physiological changes can be found in the uterine cavity, so the outcomes of postoperative 3D-TVUS can be closer to that of the uterine cavity in preparation for pregnancy than the results of preoperative 3D-TVUS. This study found that a thicker and homogeneous endometrium after HA was related to a higher live birth rate. It is suggested that more attention should be paid to evaluation of the uterine cavity, especially the endometrium, by means of 3D-TVUS to guide reproductive pursuits.
The correlation analysis found that the postoperative 3D-TVUS results were consistent with the intraoperative results of second-look hysteroscopy. Our research found that homogeneous endometrial echo was related to the no segmentation of scar contraction or no segmentation of the endometrial absence. Thicker endometrium was associated with no segmentation of the endometrial absence. 3D-TVUS is a safe and noninvasive examination, which produces results consistent with those of invasive hysteroscopy. Based on the results of this study, we recommend using noninvasive ultrasound to evaluate the uterine condition after HA, as it has more advantages than hysteroscopy, which can reduce the trauma and pain of the operation, lower the cost, and is easy to popularize. In addition, 3D-TVUS has irreplaceable advantages of hysteroscopy, such as the evaluation of uterine contour, endometrial blood flow, and diagnosis of uterine malformation (11). Therefore, for moderate-to-severe patients with IUA who have completed HA treatment, hysteroscopy is not recommended to evaluate the uterine cavity before pregnancy, but 3D-TVUS is the first choice.
We previously found that an AFS score <4 was a favorable factor for live birth (10). However, this study did not find any correlation. For the first time, 3D-TVUS parameters after the second-look hysteroscopy were included, which was closer than the last operation to the intrauterine conditions before pregnancy. The AFS score was evaluated during the last HA, and there may be recurrence of IUA after an operation. Furthermore, the AFS score cannot more clearly describe the sites of adhesion and endometrial loss, which are important to evaluate pregnancy outcomes, whereas 3D-TVUS can clearly assess those two parameters. For example, even if the AFS score is less than 4, the endometrial loss is mainly located in the upper segment of the uterine cavity, which is still not conducive to embryo implantation. When the AFS score is more than 4, the adhesions were mainly located in the lower segment of the uterus, which may have little effect on pregnancy. Therefore, the last 3D-TVUS outcomes are more practical and accurate than the AFS score in predicting pregnancy outcomes.
IUA can reduce endometrial receptivity and affect embryo implantation and development. The possible mechanism is as follows (23,29): (I) endometrial microenvironment changes can reduce endometrial receptivity; (II) the location of the lesion affects uterine contractility and embryo implantation; (III) IUA reduces the volume of the uterine cavity and interferes with sperm movement and embryo implantation; (IV) inflammation of the endometrium affects the endocrine function of the endometrium. The implantation of fertilized eggs mainly occurs in the middle or upper segment of the uterine cavity (29,30). When the adhesive tissues are located in the middle or upper segment of the uterine cavity, it may affect embryo implantation and development, thus reducing the live birth rate (23,29,30). Our study found that the lower segment of the adhesion location is a protective factor of live birth, whereas the missing part of the endometrium in the upper segment is a risk factor for live birth, confirming the findings of existing studies (23,29,30).
Moreover, the findings of this study are consistent with our previous research. ART can increase the live birth rate (9), suggesting that regardless of the number of visible tubal ostia during the second-look hysteroscopy, ART can still improve the pregnancy outcome. However, we need to recognize that it is still of important clinical value to judge the number of visible tubal ostia. For example, when the two tubal ostia are invisible, it is recommended to choose ART instead of spontaneous pregnancy. Therefore, this parameter can provide a certain value for guiding patients to choose the appropriate method of conception.
The predict model included age, endometrial thickness, pregnancy patterns, endometrial echo, segment of scar contraction, and segmentation of the endometrial absence. The nomogram indicated a younger IUA patient who has thicker endometrium, a homogeneous endometrial echo, a lower segment of scar contraction, and a lower segmentation of the endometrial absence might be easier to have a live birth by ART. The model is helpful for clinicians to give reasonable fertility guidance to IUA patients, so patients can have a better understanding of their fertility outcomes. Sometimes, unnecessary loss of time and money can be avoided. As a non-invasive and non-radiological examination, 3D-TVUS it is more easily accepted by patients during pregnancy preparation.
The research also has some limitations. The diagnosis of 3D-TVUS depends on the professional ability of sonographers and the advancement of equipment, so it is meaningful to predict the live birth rate on the premise of accurate ultrasound diagnosis. Whether the 3D-TVUS parameters will be changed dynamically with the extension of the time before pregnancy after HA and what is the best time point to perform the 3D-TVUS examination remains to be further discussed. This is a retrospective study; prospective studies are needed to further confirm the conclusions of this research.
Conclusions
In conclusion, the clinical predictive model has more practicability. With the help of noninvasive ultrasound examination, the postoperative live birth rate of patients with IUA can be predicted. Doing so can reduce the pain and economic cost of patients, and this method is easy to promote. Therefore, we suggest that 3D-TVUS be performed during the preparation for pregnancy after HA in patients with moderate-to-severe IUA.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1014/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1014/coif). All authors have reported that this study was supported by the Hunan Provincial Clinical Medical Technology Innovation Guiding Project (Nos. 2021SK53704 and 2021SK53711), the Natural Science Foundation of Hunan Province (Nos. 2021JJ40956 and 2020JJ4859), the Key Research and Development Program of Hunan province (No. 2022SK2033), and the Hunan Science and Technology Department (No. 2020 SK4017). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Third Xiangya Hospital of Central South University (IRB No. I 21053). All participants signed an informed consent form. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and all the terms related to human study participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Santamaria X, Isaacson K, Simón C. Asherman's Syndrome: it may not be all our fault. Hum Reprod 2018;33:1374-80.
- Salazar CA, Isaacson K, Morris S. A comprehensive review of Asherman's syndrome: causes, symptoms and treatment options. Curr Opin Obstet Gynecol 2017;29:249-56. [Crossref] [PubMed]
- Deans R, Vancaillie T, Ledger W, Liu J, Abbott JA. Live birth rate and obstetric complications following the hysteroscopic management of intrauterine adhesions including Asherman syndrome. Hum Reprod 2018;33:1847-53. [Crossref] [PubMed]
- Khan Z, Goldberg JM. Hysteroscopic Management of Asherman's Syndrome. J Minim Invasive Gynecol 2018;25:218-28. [Crossref] [PubMed]
- Cao M, Pan Y, Zhang Q, You D, Feng S, Liu Z. Predictive value of live birth rate based on different intrauterine adhesion evaluation systems following TCRA. Reprod Biol Endocrinol 2021;19:13. [Crossref] [PubMed]
- Zhang Y, Zhu X, Zhang T, Zhang Y, Zhang M, Lin X. Analysis of risk factors for obstetric outcomes after hysteroscopic adhesiolysis for Asherman syndrome: A retrospective cohort study. Int J Gynaecol Obstet 2022;156:89-94. [Crossref] [PubMed]
- Zhu R, Duan H, Xu W, Wang S, Gan L, Xu Q, Li J. Decision tree model predicts live birth after surgery for moderate-to-severe intrauterine adhesions. BMC Pregnancy Childbirth 2022;22:78. [Crossref] [PubMed]
- Fernandez H, Al-Najjar F, Chauveaud-Lambling A, Frydman R, Gervaise A. Fertility after treatment of Asherman's syndrome stage 3 and 4. J Minim Invasive Gynecol 2006;13:398-402. [Crossref] [PubMed]
- Sun D, Mao X, Zhang A, Gao B, Huang H, Burjoo A, Xu D, Zhao X. Pregnancy Patterns Impact Live Birth Rate for Patients With Intrauterine Adhesions After Hysteroscopic Adhesiolysis: A Retrospective Cohort Study. Front Physiol 2022;13:822845. [Crossref] [PubMed]
- Zhao X, Sun D, Zhang A, Huang H, Zhu X, Yi S, Xu D. Uterine Cavity Parameters Evaluated by Hysteroscopy can Predict the Live Birth Rate For Intrauterine Adhesion Patients. Front Med (Lausanne) 2022;9:926754. [Crossref] [PubMed]
- Zhao X, Yang Y, Liao D, Traoré A, He S, Xu D. Correlative study of preoperative three-dimensional transvaginal ultrasound findings and ongoing pregnancy/live birth in patients with intrauterine adhesions following hysteroscopic adhesiolysis: a retrospective study. Quant Imaging Med Surg 2022;12:2441-53. [Crossref] [PubMed]
- Zhao X, Gao B, Yang X, Zhang A, Jamail G, Li Y, Xu D. The density of endometrial glandular openings: a novel variable to predict the live birth rate in patients with intrauterine adhesions following hysteroscopic adhesiolysis. Hum Reprod 2021;36:965-75. [Crossref] [PubMed]
- Valle RF, Sciarra JJ. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol 1988;158:1459-70. [Crossref] [PubMed]
- Zhang A, Jamail G, Xue M, Guan X, Xiao S, Xu D. Hysteroscopic Intrauterine Adhesiolysis Using the "Ploughing" Technique With Cold Scissors. J Minim Invasive Gynecol 2015;22:934-5. [Crossref] [PubMed]
- Zhao X, Zhang A, Gao B, Burjoo A, Huang H, Xu D. Cold scissors ploughing technique in hysteroscopic adhesiolysis: a comparative study. Ann Transl Med 2020;8:50. [Crossref] [PubMed]
- Pabuçcu EG, Kovanci E, Şahin Ö, Arslanoğlu E, Yıldız Y, Pabuçcu R. New Crosslinked Hyaluronan Gel, Intrauterine Device, or Both for the Prevention of Intrauterine Adhesions. JSLS 2019;23:e2018.00108.
- Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. Endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound in patients with small intramural uterine fibroids during IVF treatment. Hum Reprod 2005;20:501-6. [Crossref] [PubMed]
- Applebaum M. The uterine biophysical profile. Ultrasound Obstet Gynecol 1995;5:67-8. [Crossref] [PubMed]
- Ijland MM, Evers JL, Dunselman GA, van Katwijk C, Lo CR, Hoogland HJ. Endometrial wavelike movements during the menstrual cycle. Fertil Steril 1996;65:746-9. [Crossref] [PubMed]
- Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, Broekmans FJ. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 2014;20:530-41. [Crossref] [PubMed]
- Gadalla MA, Huang S, Wang R, Norman RJ, Abdullah SA, El Saman AM, Ismail AM, van Wely M, Mol BWJ. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:64-76. [Crossref] [PubMed]
- Weiss NS, van Vliet MN, Limpens J, Hompes PGA, Lambalk CB, Mochtar MH, van der Veen F, Mol BWJ, van Wely M. Endometrial thickness in women undergoing IUI with ovarian stimulation. How thick is too thin? A systematic review and meta-analysis. Hum Reprod 2017;32:1009-18. [Crossref] [PubMed]
- Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell- based therapy. Biomed Pharmacother 2018;102:333-43. [Crossref] [PubMed]
- Liu KE, Hartman M, Hartman A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian Fertility and Andrology Society. Reprod Biomed Online 2019;39:49-62. [Crossref] [PubMed]
- Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther 2019;10:257. [Crossref] [PubMed]
- Benor A, Gay S, DeCherney A. An update on stem cell therapy for Asherman syndrome. J Assist Reprod Genet 2020;37:1511-29. [Crossref] [PubMed]
- Cen J, Zhang Y, Bai Y, Ma S, Zhang C, Jin L, Duan S, Du Y, Guo Y. Research progress of stem cell therapy for endometrial injury. Mater Today Bio 2022;16:100389. [Crossref] [PubMed]
- Zhang H, Xu D, Li Y, Lan J, Zhu Y, Cao J, Hu M, Yuan J, Jin H, Li G, Liu D. Organoid Transplantation Can Improve Reproductive Prognosis by Promoting Endometrial Repair in Mice. Int J Biol Sci 2022;18:2627-38. [Crossref] [PubMed]
- Bingol B, Gunenc MZ, Gedikbasi A, Guner H, Tasdemir S, Tiras B. Comparison of diagnostic accuracy of saline infusion sonohysterography, transvaginal sonography and hysteroscopy in postmenopausal bleeding. Arch Gynecol Obstet 2011;284:111-7. [Crossref] [PubMed]
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013;34:939-80. [Crossref] [PubMed]


