
The anatomical variations of paranasal sinuses may be related to the formation of antrochoanal polyp by computed tomography imaging study
Introduction
Antrochoanal polyp (ACP) is a benign lesion that develops in the maxillary sinus and has a slender stem pedicle that projects backward into the post nasal space (1). It is also known as Killian’s polyp. In 1753, Palfijn (2) first reported lesions of ACP, and then Killian described it in detail in 1906 (3).
ACPs account for about 4% of all nasal polyps (4). They are usually unilateral and can occur in females and males of any age (5). Published studies have consistently shown male preponderance; children also have a high incidence (1,6-8). The clinical symptoms of ACP are nasal obstruction and ACP-related sleep apnea (1,7). Severe cases also show other symptoms, such as cheek pain, tinnitus and hypoacusis (9). Nasal endoscopy and computed tomography (CT) are important tools for diagnosis (10). Functional endoscopic sinus surgery is the first-choice of treatment at present (11), but the recurrence rate is high, and the rate is higher in young patients (reaching 30.4%) (12), which has caused the extensive attention of otolaryngologists to the disease.
The pathogenesis of ACPs is controversial. There is a study that has previously focused on the association of ACP with anatomic variations or inflammation (9). Other scholars have explored the possible role of anatomic variations [agger nasi (AN), accessory maxillary ostium (AMO), ectopic middle turbinate (EMT), concha bullosa (CB), hyperpneumatized ethmoid bulla (HEB), nasal septal deviation (NSD), uncinate pathology (UP), and Haller cells] in the development of ACP (8,13,14). Nonetheless, the findings of their research differ. Some indicate that NSD positively influences the development of ACP (8,14), and there are views that AMO and maxillary sinus retention cyst (MSRC) do the same (13,14). To explore the possible role of them in ACP formation, we presented the concept of the nasal meatus-related parameters. We not only investigated ostiomeatal complex (OMC)-related anatomic variations, such as AN, AMO, NSD, CB, HEB, UP, and EMT, but our study also discussed the relationship among anatomic variations, maxillary sinus volume (V), nasal meatus-related parameters, and the occurrence of ACP. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1034/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shandong Provincial ENT Hospital Ethics Committee (No. 2016-KY-069) and individual consent for this retrospective analysis was waived.
Retrospective evaluations were performed on 147 patients who underwent unilateral ACP surgery from February 2010 to February 2020. All ACPs patients’ clinical data (including age, sex, laterality, concurrent disorders, and CT scans) were collected after a thorough review of the medical records, who were identified by pathological diagnosis. However, 20 patients whose CT imaging scans were unavailable or whose structural changes could not be observed on imaging were excluded. Patients with a history of sinonasal surgery and those who were unable to be evaluated were also excluded. The non-ACP side served as a control. There were 127 patients ultimately included in our cross-sectional study (Figure 1).

CT acquisition and evaluation indicators
All patients underwent CT examinations using ultradetector-row CT (Siemens Medical Systems, Berlin, Germany) at Shandong Provincial ENT Hospital. The orbits and nasal structures were scanned in helical mode at a tube voltage of 120 kV and a current of 230 mA. The 0.75 mm thick transverse scans were transformed into coronal plane CT sections that were 3 mm thick. Four specialists (two radiologists and two otolaryngologists) evaluated the images. The working years of the two otolaryngologists were 8 and 5 years, respectively, and that of the two radiologists, 5 and 4 years, respectively, who were not aware of the study endpoints.
Examination of anatomic variation in the paranasal sinus
We performed coronal, sagittal, and cross-sectional multiplanar reconstruction of CT images to evaluate the anatomic variations of the OMC and maxillary sinus-related parameters. The anatomic variations of the affected side and the opposite side were recorded. Anatomic variations included AN, AMO, EMT, CB, HEB, NSD, UP, and Haller cells (Figure 2), the following are the criteria for the identification of anatomical variation in the CT evaluation.

AN: the gas chamber that was vaporized from the bulge in the upper part of the front of the middle turbinate and caused the frontal recess to shrink was considered to be the AN (Figure 2A).
NSD: due to the fact that most people have a slight deviation of the nasal septum, we defined NSD as an angle of NSD >9° according to the grading system described by Elahi et al. (15) (Figure 2B).
CB: the gasification of the vertical lamellar or inferior globular part of the middle turbinate found in coronal or sagittal position was evaluated as CB (Figure 2B).
HEB: because the precise definition of HEB remains unclarified (16,17), we defined HEB as the distance between the upper edge of the uncinate process and the lower edge of the ethmoid vesicle and (or) the distance between the inner edge of the ethmoid vesicle and the outer wall of the middle turbinate ≤2 mm (Figure 2B).
EMT: included middle turbinate inversion (middle turbinate protruding from outside to medial) (Figure 2C) and middle turbinate dysplasia.
AMO: the opening of the maxillary sinus that appeared in the anterior or posterior fontanelle except the orifice of the maxillary sinus was recognized as the accessory orifice of the maxillary sinus (Figure 2C).
Haller cells: the cells formed by the gasification of the anterior ethmoid bone protruding into the inferior orbital wall were considered to be Haller cells (Figure 2D).
UP: inward migration of the uncinate process (the nasal septum’s vertical line and the uncinate process’s long axis form the angle <135°), outward movement of the uncinate process (the nasal septum’s vertical line and the uncinate process’s long axis form the angle >145°), and pneumatization of the uncinate process (air density in the uncinate process) (Figure 2F) were considered as UP.
Measurement of maxillary sinus- and nasal meatus-related parameters
To obtain accurate data, the incidence of MSRC was determined via pathological diagnosis and calibrated by an otolaryngologist and a radiologist, the working years of the otolaryngologist was 8 years and that of the radiologist was 4 years.
V was measured on the coronal, sagittal, and transverse planes using 3DSlicer software. The patients’ maxillary sinuses were delineated and segmented by a radiologist who separated the maxillary sinus from the nasal passages and other anatomical structures. 3D images of patients’ maxillary sinuses were reconstructed using 3DSlicer’s Segment Editor tool, and V was automatically calculated by this software (Figure 3). This process was carried out twice, and V was measured in cm3.

The maximum length from the maxillary sinus lateral wall to the nasal septum (L) was the distance from the maxillary sinus outermost wall to the reconstructed nasal septum (the standard position of the nasal septum, which was consistent with the midline of the human body) (Figure 4A).

The length from the maxillary sinus orifice to the plane of the most lateral margin of the middle turbinate (L1) was measured as follows: the horizontal distance between the highest point of the maxillary sinus medial wall and the outermost point of the bone in the ipsilateral middle turbinate’s horizontal part at the maximum level of the maxillary sinus opening (Figure 4B).
The distance from the inferior turbinate to the nasal septum (L2) was defined as follows: the horizontal distance between the outermost point of the nasal septum and the innermost point of the inferior turbinate bone at the maximum level of the maxillary sinus orifice (below the horizontal line of the highest point of the maxillary sinus medial wall) (Figure 4C).
The data for V, L, L1, and L2 were measured twice and averaged by an otolaryngologist and a radiologist whose working times were both 5 years.
Statistical analysis
SPSS 26.0 (IBM SPSS, Armonk, NY, USA) was used for statistical analysis. V and related parameters were analyzed in two groups according to age: the adult group (>18 years old) and the child group (≤18 years old). The Kolmogorov-Smirnov test was used to assess whether the data followed a normal distribution. The continuous variables that confirmed to a normal distribution were presented as mean ± standard deviation. The differences in CT evaluation indicators, V, L, L1 and L2 between groups were examined using paired t-test. The Chi-squared test was used to compare the relationship between age and sex. A two-sided P value less than 0.05 was considered statistically significant.
Results
Basic characteristics of the ACP patients in children and adults
This study comprised 127 individuals with ACP, of whom 42 (33.1%) were female and 85 (66.9%) were male. There were 45 patients in the pediatric group whose mean age was 12.11±3.26 (range, 6–18 years old), including 35 males and 10 females. In the adult group, there were 82 patients whose mean age was 43.29±15.02 (range, 19–83 years old), including 32 females and 50 males. The distribution of males and females between the two groups did not significantly differ from one another (P=0.054). ACP was identified on the right side in 21 (46.7%) patients and another side in 24 (53.3%) patients in the children group. There were 39 (47.6%) patients in the adult group having the ACP on their right side, while 43 (52.4%) patients had ACP on their left side. The laterality of ACP between the child group and the adult group did not differ significantly (P=0.923). Table 1 displays the clinical data of the patients.
Table 1
| General data | Adult (n=82) | Child (n=45) | P value |
|---|---|---|---|
| Age (years) | 43.29±15.02 [19–83] | 12.11±3.26 [6–18] | |
| Gender | 0.054 | ||
| Male | 50 (61.0) | 35 (77.8) | |
| Female | 32 (39.0) | 10 (22.2) | |
| ACP | 0.923 | ||
| Left | 43 (52.4) | 24 (53.3) | |
| Right | 39 (47.6) | 21 (46.7) |
Data are presented as the mean ± standard deviation [range] or n (%). ACP, antrochoanal polyp.
Anatomic variations in ACP patients
The anatomic variation with the highest incidence was AN (96.9%), followed by AMO (73.2%), NSD (67.7%), Haller cell (48.0%), CB (41.7%), MSRC (33.9%), and HEB (33.9%). UP (11.0%) and EMT (7.1%) were rarely observed (Table 2). Comparing the two sides, the incidence of AMO (68.5% on the ACP side versus 42.5% on the non-ACP side) and MSRC (9.4% on the ACP side versus 29.1% on the non-ACP side) were significantly different (both P<0.001). There was no significant distinction between the two sides in the prevalence of other anatomic variations.
Table 2
| CT evaluation indicators | Prevalence rate, n (%) | Number of ACP side, n (%) | Number of non-ACP side, n (%) | P value |
|---|---|---|---|---|
| AN | 123 (96.9) | 113 (89.0) | 104 (81.9) | 0.095 |
| AMO | 93 (73.2) | 87 (68.5) | 54 (42.5) | <0.001*** |
| NSD | 86 (67.7) | 47 (37.0) | 40 (31.5) | 0.450 |
| Haller cell | 61 (48.0) | 46 (36.2) | 45 (35.4) | 0.858 |
| CB | 53 (41.7) | 41 (32.3) | 48 (37.8) | 0.090 |
| MSRC | 43 (33.9) | 12 (9.4) | 37 (29.1) | <0.001*** |
| HEB | 43 (33.9) | 36 (28.3) | 30 (23.6) | 0.181 |
| UP | 14 (11.0) | 6 (4.7) | 11 (8.7) | 0.132 |
| EMT | 9 (7.1) | 5 (3.9) | 5 (3.9) | >0.999 |
***, P<0.001. P, the difference of anatomic variations incidence between the ACP and non-ACP sides. CT, computed tomography; ACP, antrochoanal polyp; AN, agger nasi; AMO, accessory maxillary ostium; NSD, nasal septal deviation; CB, concha bullosa; MSRC, maxillary sinus retention cyst; HEB, hyperpneumatized ethmoid bulla; UP, uncinate pathology; EMT, ectopic middle turbinate.
The difference in V on the affected and healthy sides of the ACP patients
The data of V and nasal meatus-related parameters have normal distribution to allow for the use of parametric tests.
In adults, the mean V of the affected side was 23.91±8.83 cm3 and that of the non-ACP side was 21.95±7.98 cm3 (P<0.001). The affected side of males was significantly larger than the healthy side (ACP side: 25.64±8.53 cm3, non-ACP side: 23.45±7.68 cm3), and there was a prominent variation (P<0.001). Additionally, the V results when contrasting the healthy (19.59±7.98 cm3) and affected (21.21±8.73 cm3) sides in women were statistically significant (P=0.011). After taking sex into account, the healthy sides (P=0.032) and the affected sides (P=0.026) were compared, as indicated in Table 3.
Table 3
| Classification | Child | Adult | |||
|---|---|---|---|---|---|
| V (cm3) | P value | V (cm3) | P value | ||
| ANT | <0.001*** | <0.001*** | |||
| ACP side | 21.80±7.65 | 23.91±8.83 | |||
| Non-ACP side | 19.29±5.94 | 21.95±7.98 | |||
| ANM | <0.001*** | <0.001*** | |||
| ACP side | 22.23±7.85 | 25.64±8.53 | |||
| Non-ACP side | 19.67±6.28 | 23.45±7.68 | |||
| ANF | 0.060 | 0.011* | |||
| ACP side | 20.28±7.10 | 21.21±8.73 | |||
| Non-ACP side | 17.94±4.54 | 19.59±7.98 | |||
| FMN | 0.422 | 0.032* | |||
| Female | 17.94±4.54 | 19.59±7.98 | |||
| Male | 19.67±6.28 | 23.45±7.68 | |||
| FMA | 0.484 | 0.026* | |||
| Female | 20.28±7.10 | 21.21±8.73 | |||
| Male | 22.23±7.85 | 25.64±8.53 | |||
Data are presented as the mean ± standard deviation. *, P<0.05; ***, P<0.001. V, maxillary sinus volume; ANT, comparison of ACP side and non-ACP side in total patients; ACP, antrochoanal polyp; ANM, comparison of ACP side and non-ACP side in male; ANF, comparison of ACP side and non-ACP side in female; FMN, comparison of female and male in non-ACP side; FMA, comparison of female and male in ACP side.
The difference was significant in the children’s group (P<0.001): the mean V of the affected side was 21.80±7.65 cm3, while that of the healthy side was 19.29±5.94 cm3. The mean V of the affected and opposite sides in males was 22.23±7.85 and 19.67±6.28 cm3, respectively (P<0.001). Moreover, the results of the comparison of the Vs of the two sides in females, Vs of the ACP sides between males and females, and Vs of the healthy sides between males and females were not significantly different (Table 3).
The difference in L between healthy and affected sides in ACP patients
In the adult group, the mean L of the ACP side was 4.43±0.32 cm in females and 4.66±0.46 cm in males (P=0.011). The mean L of the non-ACP side was found to be 4.37±0.40 cm in females and 4.62±0.51 cm in males (P=0.024). Furthermore, when the healthy and affected sides were compared separately in three groups (men, women, and all adults), there was no significant difference. In the children group, no significant differences were discovered in all data analyses. The data are shown in Table 4.
Table 4
| Classification | Child | Adult | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L (cm) | L1 (cm) | L2 (cm) | L (cm) | L1 (cm) | L2 (cm) | ||||||||||||
| Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | ||||||
| ANT | 0.192 | 0.044* | 0.590 | 0.090 | 0.014* | 0.013* | |||||||||||
| ACP side | 4.43±0.44 | 0.52±0.19 | 0.45±0.17 | 4.57±0.43 | 0.49±0.19 | 0.45±0.18 | |||||||||||
| Non-ACP side | 4.38±0.42 | 0.46±0.14 | 0.44±0.12 | 4.52±0.48 | 0.55±0.15 | 0.51±0.19 | |||||||||||
| ANM | 0.620 | <0.001*** | 0.921 | 0.270 | 0.069 | <0.001*** | |||||||||||
| ACP side | 4.46±0.46 | 0.50±0.17 | 0.44±0.14 | 4.66±0.46 | 0.50±0.18 | 0.44±0.15 | |||||||||||
| Non-ACP side | 4.44±0.42 | 0.46±0.14 | 0.44±0.13 | 4.62±0.51 | 0.55±0.13 | 0.56±0.19 | |||||||||||
| ANF | 0.448 | 0.159 | 0.448 | 0.189 | 0.097 | 0.615 | |||||||||||
| ACP side | 4.31±0.35 | 0.59±0.25 | 0.49±0.24 | 4.43±0.32 | 0.47±0.21 | 0.46±0.23 | |||||||||||
| Non-ACP side | 4.19±0.33 | 0.44±0.12 | 0.44±0.12 | 4.37±0.40 | 0.55±0.18 | 0.43±0.16 | |||||||||||
| FMN | 0.089 | 0.656 | 0.988 | 0.024* | 0.976 | 0.003** | |||||||||||
| Female | 4.19±0.33 | 0.44±0.12 | 0.44±0.12 | 4.37±0.40 | 0.55±0.18 | 0.43±0.16 | |||||||||||
| Male | 4.44±0.42 | 0.46±0.14 | 0.44±0.13 | 4.62±0.51 | 0.55±0.13 | 0.56±0.19 | |||||||||||
| FMA | 0.362 | 0.214 | 0.508 | 0.011* | 0.464 | 0.742 | |||||||||||
| Female | 4.31±0.35 | 0.59±0.25 | 0.49±0.24 | 4.43±0.32 | 0.47±0.21 | 0.46±0.23 | |||||||||||
| Male | 4.46±0.46 | 0.50±0.17 | 0.44±0.14 | 4.66±0.46 | 0.50±0.18 | 0.44±0.15 | |||||||||||
*, P<0.05; **, P<0.01; ***, P<0.001. L, the maximum length from the lateral wall of the maxillary sinus to the nasal septum; L1, the length from the maxillary sinus orifice to the plane of the most lateral margin of the middle turbinate; L2, the minimum length from the inferior turbinate to the nasal septum; SD, standard deviation; ANT, comparison of ACP side and non-ACP side in total patients; ACP, antrochoanal polyp; ANM, comparison of ACP side and non-ACP side in male; ANF, comparison of ACP side and non-ACP side in female; FMN, comparison of female and male in non-ACP side; FMA, comparison of female and male in ACP side.
The difference in L1 between healthy and affected sides in ACP patients
The mean L1 of the ACP sides in the adults was 0.49±0.19 cm, while that of the non-ACP sides was 0.55±0.15 cm (P=0.014). The results of the other four analyses were not significantly different, as indicated in Table 4.
In the child group, the mean L1 on the ACP side was 0.52±0.19 cm, whereas the mean L1 of the non-ACP side was 0.46±0.14 cm (P=0.044). In males, the mean L1 was 0.50±0.17 cm on the ACP side, and the corresponding value was 0.46±0.14 cm on the non-ACP side (P<0.001). The results of the other three analyses were also not statistically significant, as indicated in Table 4.
The difference in L2 between healthy and affected sides in ACP patients
In the adult group, the L2 on the ACP sides was 0.45±0.18 cm and that on the non-ACP sides was 0.51±0.19 cm (P=0.013). On the non-ACP side, the mean L2 was 0.56±0.19 cm of males, whereas on the ACP side, it was 0.44±0.15 cm (P<0.001). The mean L2 of males was 0.56±0.19 cm, while that of females was 0.43±0.16 cm (P=0.003). Furthermore, as demonstrated in Table 4, no significant difference was discovered when contrasting the results of the other two analyses. And there was no significant difference in all analyses in children.
Discussion
The anatomic variations of the OMC have been assumed to be one of the risk factors for paranasal sinus disease (18). It has been found that anatomic variation might be related to the formation of ACPs because it could lead to the closure of the OMC and the increase in maxillary sinus pressure (1,5,19,20). We investigated OMC-related anatomic variations to explore their possible role in ACP formation. The results showed that the most common anatomic variations on the ACP sides were as follows: AN (89.0%), AMO (68.5%) and NSD (37.0%). In the previous study, the most common anatomic variations on the ACP sides were AN (87.0%), AMO (74.1%), and CB (42.6%) (13). Although AN, AMO, and NSD all have high prevalence, only AMO was significantly different between the healthy and affected sides of ACP patients in our study.
AMO could be an acquired deficiency caused by paranasal sinus disorders, such as sinusitis (21). In all patients with AMO on the ACP sides (87 patients), the major part of ACPs extended outside the maxillary sinus through the AMO. In other studies, the incidence of AMO on the affected side was 74.0–97.5% (7,19), which was significantly higher than that in the healthy population (20.6%) (14). When there are two drain entrances (the maxillary ostium and the AMO) of the maxillary sinus, the airflow exacerbates the phenomenon that the MSRC enters the middle meatus through the AMO, according to Bernoulli’s theorem (1). Thus, the emergence of AMO may be one of the anatomical bases of ACPs.
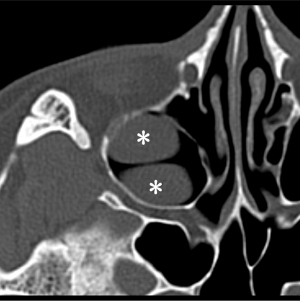
The incidence of MSRC in people with AMO is approximately three times higher than that in healthy people (22). It is interesting in our study that the incidence of MRSC on the affected side was lower than that on the healthy side in ACP patients. The reason for this phenomenon may be that the retention cyst on the affected side have developed into ACPs (23), leading to the classification of the retention cyst as the cystic part of ACPs on CT images. Therefore, MSRC might be the pathological origin of ACP formation. In addition, we found 12 patients (9.4%) in whom the retention cysts were on the ACP side, indicating that multiple cysts may be present on the ACP side at the same time (Figure 5).
The anatomical variation has a positive effect on the development of ACP by influencing nasal ventilation (13,14), and our findings support this view. To further investigate the role of nasal airflow in ACP formation, we measured the size of the nasal space (V, L, L1, and L2). Nasal meatus-related parameters could more intuitively reflect the impact of anatomical variations on the nasal meatus, and combined with statistics of anatomic variations, they would more comprehensively support our hypothesis.
The mean V of adults is larger than children because the sinuses of children are not mature and are smaller than those of adults, which is in line with the characteristics of sinus development (24). Because Vs were unaffected by maxillary sinusitis (24,25) and the major V of the patients’ affected side was larger than that of healthy side, we speculated that an increase in V may be connected to ACP formation.
L reflects the overall size of the nasal structure. Since the majority of airflow into and out of the nose passes through the inferior and middle nasal cavities (26), we measured L1 and L2 to reflect the width of the nasal meatus in different positions. In evaluating the effects of sexual factors on V and nasal meatus-related parameters (L, L1, and L2), as well as their differences between the ACP and non-ACP sides, we made the following assumptions: first, the overall measured values for males would be significantly larger than those of females; second, because the lateral wall of the maxillary sinus is thicker and more durable than other walls, it is less damaged, and the difference in L between ACP and non-ACP sides would be less; third, because of different anatomic variations on the nasal meatus and the substantial increase in the volume of the affected side, L1 and/or L2 on the non-ACP side would be considerably larger than that on the affected side. So, we established a corresponding validation estimation model: L ≈ L1 + L2 + R (transverse diameter of the maxillary sinus).
We discovered differences in L1 between the child and adult groups. L1 identified that the middle nasal cavity was becoming narrower. One of the reasons for inflammatory illnesses of the nasal meatus is nasal meatus constriction, which also results in quicker airflow through the region. Since ACP is also an inflammatory disease, a decrease in L1 may contribute to the development of ACP. Our hypothesis was consistent with the result that the L1 on the ACP side in adults is smaller than that on the healthy side, but L1 on the ACP side was larger than that on the opposite side in children. We speculated that the ACP might press the middle turbinates medially during its growth from the maxillary sinus into the nasal cavity, increasing the distance of L1 in children. Although the L1 on the affected side was larger than that on the healthy side in children, the L1 of adults on the affected side was still larger than that of children. So, ACP in adult would not cause the middle turbinate to move inward to increase L1’s length. In male children, this finding was particularly noticeable. A reduced L1 on the affected side causes a narrowing of the nasal space, an increase in air velocity, and an increase in nasal passage resistance, all of which may contribute to the development of ACP. In the adult group, especially in male patients, L2 was substantially smaller on the ACP side than on the opposite side. The narrowing of the common nasal meatus was reflected in the L2 decrease on the ACP side. The degree of nasal stenosis was exacerbated in conjunction with the reduction in L1 and L2. This resulted in increasing the velocity and pressure of airflow entered the maxillary sinus.
The bilateral V, bilateral L, and healthy side L2 of males were significantly larger than those of females, reflecting the impact of sex on nasal meatus-related data and V. This phenomenon was consistent with the fact that the skulls of males are larger than those of females. We found that the maxillary sinus on the ACP side grew in volume and transverse diameter together with the related anatomic variations, leading to the reduction of L1 and L2, thus affecting the trajectory of nasal airflow. Combined with the knowledge of hydrodynamics, we hypothesize that the occurrence of ACP requires both the OMC-related anatomic variations as an anatomical basis and the MSRC as a pathological basis, which alters the nasal airflow and leads to the formation and development of ACP. This coincides with the views of Garaycochea et al. (9). Based on the presence of the AMO, the V increase led to stenosis of corresponding channels around the OMC and the common meatus, the velocity of air flow and the pressure are increased in the nasal cavity and the maxillary sinus. The MSRC enters the middle meatus through the AMO into the nasal cavity and even the choana on high pressure which eventually develop into ACPs. The point of Frosini et al. (1) was consistent with ours. While the growth of ACPs may lead to the enlargement of the maxillary sinus, which further leads to the narrowing of the nasal cavity, positive feedback creates a multi-inflammatory environment suitable for the growth of ACPs. Li et al. also noted that there were significant differences in maximum airflow velocity, minimal airflow temperature, maximum wall shear stress, and nasal resistance between the deviated side (the narrow side of the nasal meatus) and the nondeviated side of the nasal septum in their hydrodynamic analysis of patients with nasal septum deviation (27). Tretiakow et al. said that the complex nasal meatus structure caused by anatomic variations would change continuous airflow in the healthy nasal cavity so that it becomes volatile (28). The findings of Li et al. revealed a positive correlation between the change in nasal volume and nasal airflow (27), which also supported our hypothesis that the change of nasal airflow is related to ACP formation.
The V on the affected and contralateral sides was measured in this study to better understand the relationship among anatomic variations, V, and ACPs. The anatomic variations affecting the OMC and maxillary sinus pressure were statistically studied on the ACP and opposite sides. In addition, this was the first study in which the L, L1, and L2 were examined to observe changes in the nasal meatus. These data can directly reflect the changes in the nasal meatus and be used to show its impact on ACPs. The small sample size and lack of systematic grouping evaluation of anatomic variation combinations (such as NSD and CB) were the study’s limitations. Additional research is needed to explain the effect of anatomic variations on the formation of ACPs.
Conclusions
In our study, the occurrence of the AMO, the maxillary sinus’s expanded size, and the stenosis of the associated channels around the OMC and common meatus are regarded as probably connected to the formation of ACPs. In addition, the anatomic variations that involve the OMC and may lead to a change in maxillary sinus pressure and nasal ventilation are important factors in the formation of ACPs.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1034/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1034/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shandong Provincial ENT Hospital Ethics Committee (No. 2016-KY-069) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frosini P, Picarella G, De Campora E. Antrochoanal polyp: analysis of 200 cases. Acta Otorhinolaryngol Ital 2009;29:21-6.
- Palfijn J, Cavelier G. Anatomie chirurgicale. Paris: Cavelier, Guillaume; 1753.
- Killian G. The origin of choanal polypi. The Lancet 1906;168:81-2.
- Sirola R. Choanal polyps. Acta Otolaryngol 1966;61:42-8.
- Al-Balas HI, Farneti P, Bellusci A, Crocetta FM, Sollini G, Pasquini E. A comparison of two endoscopic techniques for the treatment of antrochoanal polyps. Acta Otorhinolaryngol Ital 2020;40:290-6. [Crossref] [PubMed]
- El-Sharkawy AA. Endoscopic management of paediatric antrochoanal polyp: our experience. Acta Otorhinolaryngol Ital 2013;33:107-11.
- Balikci HH, Ozkul MH, Uvacin O, Yasar H, Karakas M, Gurdal M. Antrochoanal polyposis: analysis of 34 cases. Eur Arch Otorhinolaryngol 2013;270:1651-4. [Crossref] [PubMed]
- Hekmatnia A, Shirvani F, Mahmoodi F, Hashemi M. Association of anatomic variations with antrochoanal polyps in paranasal sinus computed tomography scan. J Res Med Sci 2017;22:3. [Crossref] [PubMed]
- Garaycochea O, Van Strahlen CR, Alobid I, Mullol J. Pheno-Endotyping Antrochoanal Nasal Polyposis. Curr Allergy Asthma Rep 2023;23:165-80. [Crossref] [PubMed]
- Ferjaoui M, Bergaoui E, Koubaa J. Atypical presentation of a polyp of Killian. Rev Med Liege 2023;78:196-8.
- Bakshi SS, Vaithy K A. Antrochoanal Polyp. J Allergy Clin Immunol Pract 2017;5:806-7. [Crossref] [PubMed]
- Pagella F, Emanuelli E, Pusateri A, Borsetto D, Cazzador D, Marangoni R, Maiorano E, Zanon A, Cogliandolo C, Ciorba A, Pelucchi S. Clinical features and management of antrochoanal polyps in children: Cues from a clinical series of 58 patients. Int J Pediatr Otorhinolaryngol 2018;114:87-91. [Crossref] [PubMed]
- Başer E, Sarıoğlu O, Arslan İB, Çukurova İ. The effect of anatomic variations and maxillary sinus volume in antrochoanal polyp formation. Eur Arch Otorhinolaryngol 2020;277:1067-72. [Crossref] [PubMed]
- Gursoy M, Erdogan N, Cetinoglu YK, Dag F, Eren E, Uluc ME. Anatomic variations associated with antrochoanal polyps. Niger J Clin Pract 2019;22:603-8. [Crossref] [PubMed]
- Elahi MM, Frenkiel S. Septal deviation and chronic sinus disease. Am J Rhinol 2000;14:175-9. [Crossref] [PubMed]
- Laine FJ, Smoker WR. The ostiomeatal unit and endoscopic surgery: anatomy, variations, and imaging findings in inflammatory diseases. AJR Am J Roentgenol 1992;159:849-57. [Crossref] [PubMed]
- Shpilberg KA, Daniel SC, Doshi AH, Lawson W, Som PM. CT of Anatomic Variants of the Paranasal Sinuses and Nasal Cavity: Poor Correlation With Radiologically Significant Rhinosinusitis but Importance in Surgical Planning. AJR Am J Roentgenol 2015;204:1255-60. [Crossref] [PubMed]
- Bilge T, Akpinar M, Mahmutoğlu AS, Uçak I, Uslu Coşkun B. Anatomic Variations in Paranasal Sinuses of Patients With Sinonasal Polyposis: Radiological Evaluation. J Craniofac Surg 2016;27:1336-9. [Crossref] [PubMed]
- Kizil Y, Aydil U, Ceylan A, Uslu S, Baştürk V, İleri F. Analysis of choanal polyps. J Craniofac Surg 2014;25:1082-4. [Crossref] [PubMed]
- Yaman H, Yilmaz S, Karali E, Guclu E, Ozturk O. Evaluation and management of antrochoanal polyps. Clin Exp Otorhinolaryngol 2010;3:110-4. [Crossref] [PubMed]
- Orhan Soylemez UP, Atalay B. Investigation of the accessory maxillary ostium: a congenital variation or acquired defect? Dentomaxillofac Radiol 2021;50:20200575. [Crossref] [PubMed]
- Yenigun A, Fazliogullari Z, Gun C, Uysal II, Nayman A, Karabulut AK. The effect of the presence of the accessory maxillary ostium on the maxillary sinus. Eur Arch Otorhinolaryngol 2016;273:4315-9. [Crossref] [PubMed]
- Berg O, Carenfelt C, Silfverswärd C, Sobin A. Origin of the choanal polyp. Arch Otolaryngol Head Neck Surg 1988;114:1270-1. [Crossref] [PubMed]
- Aşantoğrol F, Coşgunarslan A. The effect of anatomical variations of the sinonasal region on maxillary sinus volume and dimensions: a three-dimensional study. Braz J Otorhinolaryngol 2022;88:S118-27. [Crossref] [PubMed]
- Pérez Sayáns M, Suárez Quintanilla JA, Chamorro Petronacci CM, Suárez Peñaranda JM, López Jornet P, Gómez García F, Guerrero Sánchez Y. Volumetric study of the maxillary sinus in patients with sinus pathology. PLoS One 2020;15:e0234915. [Crossref] [PubMed]
- Lee TS, Goyal P, Li C, Zhao K. Computational Fluid Dynamics to Evaluate the Effectiveness of Inferior Turbinate Reduction Techniques to Improve Nasal Airflow. JAMA Facial Plast Surg 2018;20:263-70. [Crossref] [PubMed]
- Li L, Zang H, Han D, Ramanathan M Jr, Carrau RL, London NR Jr. Impact of a Concha Bullosa on Nasal Airflow Characteristics in the Setting of Nasal Septal Deviation: A Computational Fluid Dynamics Analysis. Am J Rhinol Allergy 2020;34:456-62. [Crossref] [PubMed]
- Tretiakow D, Tesch K, Markiet K, Skorek A. Maxillary sinus aeration analysis using computational fluid dynamics. Sci Rep 2022;12:10376. [Crossref] [PubMed]


