
Quadricuspid pulmonic valve with pulmonary artery aneurysm
Introduction
Quadricuspid pulmonary valve (QPV) is a rare congenital malformation. The European Homograft Bank (EHB), an international organization of European countries, analyzed nearly 20 years of data from cooperating institutions since 1989 and identified eight cases of QPV in 3,861 donor hearts (1). Pulmonary aneurysm is also associated with low morbidity and rarely diagnosed. The majority of cases of pulmonary aneurysm are diagnosed incidentally and present asymptomatically, and only a few cases of pulmonary aneurysm in combination with a 4-lobe pulmonary valve have been reported previously.
Case presentation
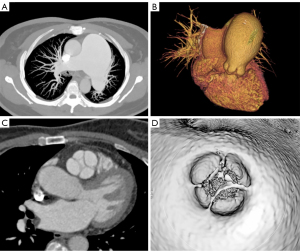
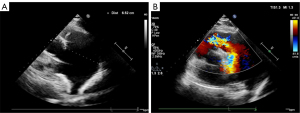
A 64-year-old woman with a history of treatment of hypertension was admitted to hospital with palpitations and dyspnea during exertion for 2 months. Physical examination showed a grade 2 systolic murmur in the left upper sternal border, without other anomalies. Electrocardiogram (ECG) showed sinus rhythm, and a preoperative coronary computed tomography (CT) showed a right dominant coronary artery with no definite stenosis in any major coronary artery segment. A dilated pulmonary artery (PA) had been incidentally discovered on a CT scan for an adrenal occupancy 2 years prior; however, due to the absence of symptoms, further investigations and treatments were not possible at the time. The admission blood pressure was 140/83 mmHg and the heart rate was 72 beats/min. After admission, CT of the PA (CTPA) showed a 4-valve malformation with a slightly thickened valve leaflet, a pulmonary sinus diameter of approximately 38 mm, and an annulus of approximately 30 mm. Aneurysmal dilatation of the main PA with a transverse diameter of 65 mm (34 mm transverse diameter of the ascending aorta at the same level), enlarged right ventricle (RV), and incomplete closure of the pulmonary valve were also observed. A CT virtual endoscopy showed the four lobes of the pulmonary valve, with the additional reconstruction of the trunk and pulmonary branches from CT 3-dimensional (3D) images (Figure 1). The ECG showed that there was no stenosis of her pulmonary valve, however, the patient had an antegrade flow velocity of 1.7 m/s, and her anteroposterior right ventricular diameter was 32 mm. Color Doppler flow imaging showed massive regurgitation in the pulmonary valve (Figure 2). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Intraoperatively, four pulmonary valves were observed, with thickened, soft edges (Figure 3). The quadricuspid pulmonary valve was removed via a PA incision and the pulmonary valve was replaced with a 27-gauge bioprosthetic valve with continuous sutures. The widened main PA and the right and left pulmonary arteries were replaced using Dacron artificial vessels. We also performed histology of the resected pulmonary trunk during the postoperative period. According to the report, the whole layer of the PA wall was thinned, with sparse elastic fibers in the media and the presence of cystic medial necrosis, indicating mild medial degeneration of the PA wall. The pathology report showed that in this case, the pulmonary trunk aneurysm was in keeping with atrophy of the elastic lamellar units in the tunica media of the PA, not with pulmonary valve regurgitation (PR).

The patient recovered successfully. The implanted valve functioned well and there was no significant perivalvular leakage postoperatively. ECG revealed that the anteroposterior diameter of the RV was 24 mm. The tricuspid annular plane systolic excursion (TAPSE) was normal, and the anteroposterior flow velocity of the pulmonary valve was 2.2 m/s. On follow-up (6 months), the correction proved stable.
Discussion
QPV can lead to severe PR, along with severe enlargement of the RV and pulmonary artery aneurysm (PAA) due to increased preload of the RV. Therefore, correction of PR helps to reduce the overload volume of the RV and the hemodynamic burden of the PA. Surgical valve replacement and pulmonary angioplasty are commonly used to prevent RV dysfunction and PAA rupture.
However, there is a lack of consensus on the optimal timing of surgical treatment for PAA. Kreibich et al. (2) recommend surgery for PAA >5.5 cm. PAA is also associated with a risk of PA entrapment or rupture, left coronary artery trunk compression, right PA thrombosis and pulmonary compression atelectasis and collapse. Mastroroberto (3) supports the indication for surgery in all cases meeting at least one of the following conditions: (I) reasonable surgical risk [excluding some cases of severe pulmonary hypertension (PHT)], (II) progressive increase in aneurysm diameter, and (III) PA entrapment.
Cardiovascular imaging studies showed that the patient’s QPV consisted of three similar cusps and one small, dysplastic cusp, which corresponded to the most common type described in the literature (4). 3D transesophageal echocardiography (TEE) clearly showed the morphological structure and motion of the QPV, providing information about the function of the abnormal valve, contributed to the preoperative decision making. Cardiac CT allows adequate characterization of valve morphology and plays a decisive role in the in vivo diagnosis of this rare congenital heart disease. The use of CT simulation endoscopy in patients with QPV, with clear visualization with computer image processing and virtual reality technology, which can provide accurate imaging support to surgeon.
Acknowledgments
Funding: The study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-898/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jashari R, Van Hoeck B, Goffin Y, Vanderkelen A. The incidence of congenital bicuspid or bileaflet and quadricuspid or quadrileaflet arterial valves in 3,861 donor hearts in the European Homograft Bank. J Heart Valve Dis 2009;18:337-44.
- Kreibich M, Siepe M, Kroll J, Höhn R, Grohmann J, Beyersdorf F. Aneurysms of the pulmonary artery. Circulation 2015;131:310-6. [Crossref] [PubMed]
- Mastroroberto P, Chello M, Zofrea S, Del Negro G, De Francesca F, Maltese G. Pulmonary artery aneurysm. Ann Thorac Surg 1997;64:585-6.
- Hurwitz LE, Roberts WC. Quadricuspid semilunar valve. Am J Cardiol 1973;31:623-6. [Crossref] [PubMed]


