
Step-by-step transesophageal echocardiographic guidance for transjugular transcatheter tricuspid valve replacement with a radial force-independent bioprosthesis
Transcatheter solutions for tricuspid regurgitation (TR)
The tricuspid valve (TV) was historically referred to as the forgotten valve and TR was usually left untreated until recent research unveiled its detrimental impact on prognosis (1). The prohibitive risk of isolated TV surgery encouraged rapid development of a transcatheter solution for TR (2), which demonstrated favorable outcomes (3,4). In recognition of this, recent updates in guidelines recommend considering transcatheter treatment of symptomatic secondary severe TR in inoperable patients (5,6).
Currently available transcatheter solutions for TR can be categorized into repair and replacement devices. To date, three of the transcatheter TV repair (TTVR) systems have been approved for commercial use, aiming at leaflet approximation [TriClip (Abbott Laboratories, Chicago, IL, USA) and PASCAL (Edward Lifesciences, Irvine, CA, USA)] and direct annuloplasty (Cardioband; Edward Lifesciences), respectively (7). For replacement devices, in addition to heterotopic valves aiming to relieve venous congestion (TricValve; OrbusNeich, Shatin, Hong Kong), several orthotopic replacement systems are under clinical and preclinical investigation. Among them, 4 of the devices [NaviGate (NaviGate Cardiac Structures, Lake Forest, CA, USA), EVOQUE (Edwards Lifesciences), LuX-Valve (Jenscare Biotechnology, Ningbo, China), and Lux-Valve Plus (Jenscare)] have published first-in-human results (8-11). These devices rely on either radial force (NaviGate, EVOQUE), tricuspid leaflet engagement (all four devices), or septal insertion (LuX-Valve and Lux-Valve Plus) for implantation and stability. The device characteristics and mechanisms of deployment are summarized in Table 1.
Table 1
| Characteristics | NaviGate | EVOQUE | LuX-Valve | LuX-Valve Plus |
|---|---|---|---|---|
| Size, mm | 36, 40, 44, 48, 52 | 44, 48, 52 | 50, 60, 70 (annular) | 50, 60, 70 (annular) |
| Anchoring mechanism | Atrial winglets and ventricular graspers | Intra-annular sealing skirt and leaflet graspers | Anterior leaflet graspers and septal anchor | Anterior leaflet graspers and septal anchor |
| Radial force | Dependent | Dependent | Independent | Independent |
| Access | Atrial (jugular approach abandoned) | Femoral | Atrial | Jugular |
| Delivery system size, Fr | 42 | 28 | 32 | 32 |
The transjugular delivery and implantation of the Lux-Valve Plus system requires intensive echocardiographic guidance and monitoring. Its unique radial force-independent design also makes the intraprocedural imaging different from other currently available TTVR systems (Figure 1). However, the imaging needs in the intraoperative guidance and immediate postoperative evaluation of this device have yet to be described.

This practice review thus aims to elaborate how 2D and 3D transesophageal echocardiography (TEE) are used to guide the implantation of the Lux-Valve Plus system and discusses the imaging requirements of this novel procedure.
Guiding the super stiff guidewire and the delivery system into the right ventricle (RV)
From the 3D mid-esophageal (ME) bicaval view, the superior vena cava (SVC) and the tricuspid orifice are displayed. Clockwise or counterclockwise rotation of the probe will allow visualization of the tricuspid orifice in a 3D en face view. The super stiff guidewire is first guided into the RV (Figure 2), and then the delivery system is inserted along the super stiff guidewire into the RV (Figure 3). During this process, the end of the delivery catheter should always be clearly displayed to avoid damage to the surrounding tissue and the super stiff guidewire is withdrawn after ensuring that the delivery catheter is at a suitable depth into the RV.


Adjusting the delivery system to align coaxially or parallel to the central axis of the tricuspid annulus plane
From the ME 4-chamber (4C) view, the X-plane mode and the 3D mode were activated to guide the delivery system coaxially with the central axis of the tricuspid annulus (Figure 4). During the adjustment, the end of the delivery system should be clearly imaged to avoid contact with surrounding structures or wrapping around the chordae tendineae, which could lead to ventricular wall perforation or tendon rupture.

Coaxiality is a common requirement for transcatheter valve implantation to avoid paravalvular leak and dislodgement (12). This is usually confirmed using two longitudinal orthogonal planes across the annular plane, which can be easily achieved for the mitral valve through the X-plane mode with the commissural view as the primary view (13). For the TV, if the abovementioned planes are insufficient to confirm coaxiality, multi-planar reconstruction can also be used (Figures S1,S2).
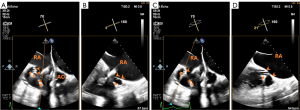
Confirming the two graspers are holding and clamping the anterior TV leaflet from the right ventricular side (critical step)
From the ME aortic valve (AV) short axis (SAX) view, slight ante- or retro-flex and clockwise or counter-clockwise rotation of the probe until the 2 rabbit-ear-like graspers of the Lux-Valve Plus can be visualized. The sample line is then placed on the graspers, and the X-plane mode is activated to monitor the opening of the graspers. It is important to confirm that the leaflet lies to the atrial side of both graspers. This could be judged from the limited motion of the anterior leaflet (Figure 5). Leaflet engagement should be carefully assessed as it is one of the major mechanisms of anchoring (9). This is especially important for the LuX-Valve system as it only has two graspers as opposed to the nine graspers on the EVOQUE valve or 12 tines on the NaviGate valve (14). In addition, 2D imaging is preferred over 3D to confirm leaflet engagement due to its better spatial resolution. Of note, the anterior leaflet is not securely clutched at this step as it requires the valve stent to expand on the atrial side of the leaflet after unsheathing to finish the clamp.
Opening of the valve disc under 3D en face view of the tricuspid orifice
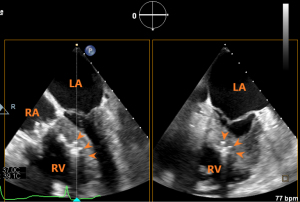
After the anterior TV leaflet is grasped, the atrial valve disc is opened and adjusted under the monitoring of a 3D en face view of the tricuspid orifice, which is obtained from the 2D ME bicaval view. The orientation of the disc is slightly tilted so that the valve stent fits the tricuspid annulus, particularly the septal portion of the annulus (Figure 6). The prosthetic valve would have been functioning at this time and the paravalvular leak can be assessed in multiple views using 2D color Doppler flow imaging (CFI) (Figure 7). It should be noted that major reposition of the valve is not possible after the expansion of the valve stent and the opening of the atrial flange because the self-expandable valve stent would approximate the leaflet graspers and clutch the leaflets within after release. This is similar to the EVOQUE system, where the anchor tips become positioned subannular to capture the leaflets as the valve is exposed and expands (15). Slight ante-, retro-, left-, or right-flex of the delivery catheter may allow better contact of the atrial flange with the underlying tissue and consequently better seal.


ME 4C X-plane view to guide and monitor the deployment of the interventricular septal anchor (critical step)
Using the ME 4C view, the interventricular septal anchor piece is adjusted to be parallel to the posterior segment of the interventricular septum (IVS). The orthogonal plane of the X-plane mode is used to ensure that the anchor piece clings to the septum and to monitor the insertion of the tip of the anchor piece (Figures 8-10). If the anchors are shown to be parallel to the IVS and not in contact with the posterior septum in the non-standard ME 4C view but contacting the posterior septum in the orthogonal right ventricular SAX view, it is then necessary to retroflex the probe to obtain the standard ME 4C and right ventricular SAX views (Figures S3,S4). If the standard ME 4C X-plane views demonstrate a nonparallel septal anchor, the delivery catheter can only be slightly rotated clockwise or counterclockwise until it is parallel to and opposing the septum to prevent leaflet tear.


Evaluation of the prosthetic valve function
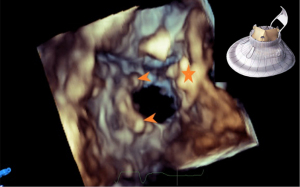
From a 3D en face view of TV, the coaxiality of the prosthetic valve and the morphology of the valve disc could be assessed. The 2D and 3D CFI from this view could be used to assess the presence and location of paravalvular leak. Paravalvular TR quantification is preferably performed through direct planimetry of the vena contracta area under 3D CFI (16), as the regurgitant orifice is irregular and the hemispheric assumption of the proximal isovelocity surface area method is violated. Severity of the paravalvular leak can be graded as per guideline recommendation (17). Angiography can further confirm the presence and severity of paravalvular leak. Rotating the 3D en face view of TV to the ventricular side of the tricuspid orifice allows visualization of the 2 graspers and the septal anchor (Figure 11).
The maximal and mean transvalvular pressure gradient should also be assessed, though elevated pressure gradient is rare (18). If the prosthetic valve is coaxial, the paravalvular leak is moderate or less, the mean transvalvular pressure gradient is less than 2 mmHg, and no other complications such as pericardial effusion are found, the valve and delivery system can be dissociated and the delivery system can be withdrawn.
Discussion
Advantages and disadvantages of 2D and 3D echocardiography
The major steps and views of Lux-Valve Plus implantation are summarized in Table 2. During each step of the LuX-Valve Plus implantation, different imaging needs entail different imaging modalities. For steps that involve definition of the general position of the device, such as confirming the coaxiality or introducing the delivery system, both 2D and 3D echocardiography can be used. However, when finer monitoring of anatomical structures or device details is needed, such as confirming that the leaflet lies to the atrial side of the two graspers or the insertion of the tip of the anchor piece into the IVS, 2D echocardiography is preferred due to its better spatial and temporal resolution. For steps requiring real-time imaging of the entire device, such as the opening of the valve disc, 3D echocardiography is the better choice. 3D echocardiography should also be used when definition of specific leaflet is hard to achieve under 2D echocardiography (19).
Table 2
| Step | TEE views | Angle ranges | Notes |
|---|---|---|---|
| 1. Guiding the super stiff guidewire and the delivery system into the right ventricle | 2D and 3D ME bicaval view | 70–110º | – |
| 3D adjusted ME bicaval view | 70–110º | Slight clockwise/counter-clockwise rotation of the probe from the ME bicaval view for appropriate depth into the TA | |
| 2. Adjusting the delivery system to align coaxially or parallel to the central axis of the tricuspid annulus plane | X-plane view with ME 4C view as the primary view | ~0º | The end of the delivery system should be clearly imaged to avoid contact with surrounding structures |
| 3D view simultaneously activated | ~0º | The depth provided by 3D view helps definition of the end of the delivery system and avoid contact with surrounding structures. Further confirmation of the coaxiality can also be done | |
| Multi-planar reconstruction | This mode could be considered in experienced centers for better definition of the TA plane and thus better coaxiality | ||
| *3. Confirming the two graspers are holding and clamping the anterior TV leaflet from the right ventricular side | X-plane view with the adjusted ME AV SAX view as the primary view | ~60º | From the ME AV SAX view, slight ante- or retro-flex and clockwise or counter-clockwise rotation of the probe until the two rabbit-ear-like graspers of the Lux-Valve Plus can be visualized |
| 4. Opening of the valve disc under 3D en face view of the tricuspid orifice | 3D en face view of the tricuspid orifice | 70–110º | Slight clockwise/counter-clockwise rotation of the probe from the ME bicaval view |
| Multiple views with 2D color Doppler flow | Assess paravalvular leak | ||
| *5. Guide and monitor the deployment of the interventricular septal anchor | X-plane view with ME 4C view as the primary view | ~0º | Ensure the anchor piece clings to the septum |
| 6. Evaluation of the prosthetic valve function | Multiple views required | – | Assess coaxiality, paravalvular leak and transvalvular pressure gradient |
*, critical steps. 2D, 2-dimensional; 3D, 3-dimensional; ME, mid-esophageal; 4C, 4-chamber; TA, tricuspid annulus; AV, aortic valve; SAX, short axis; TV, tricuspid valve.
Future technical improvements
Current 3D technology has been shown to be able to deliver accurate chamber quantification from the deconstructed 2D views (20). Future improvement in spatial and temporal resolution of 3D echocardiography may allow accurate surveillance of much finer anatomical and device details that could significantly reduce the views needed for intraoperative guidance.
All current transcatheter TV replacement systems with published human results are not repositionable. Next-generation devices with recapturable design including the Trisol and the Intrepid system (NCT04433065) may improve the possibility of successful implantation (18).
Limitations
Several limitations of the current study should be addressed. Firstly, the transgastric projections are very useful views to illustrate the TV but are not used during the guidance. Such imaging strategy was taken to prevent accidental bleeding events. Our initial experience involves mainly end-stage patients who often may present with visceral congestion and gastric varices. The time limits in the catheterization lab may cause an anxious echocardiographer to mistakenly pull back the TEE probe without restoration into a neutral position, which could be catastrophic in such patients. The transgastric projections were thus avoided during the guidance but are only reserved for pre-procedural planning in our center. However, the authors do agree that the transgastric projections are important views for identifying the TV leaflets. In experienced centers, these views could be incorporated into the imaging protocol to help intra-operative decision making. Secondly, the proposed protocol is designed for teams that are beginning and/or learning how to perform the Lux-Valve Plus implantation to reduce the time needed for the proficiency of the echocardiographers and the interventionists. The simplified TEE monitoring regimen is not a substitute for the comprehensive knowledge and imaging protocols covered in current guidelines (17,21,22).
Acknowledgments
Funding: This research was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-218/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by institutional ethics board of Zhongshan Hospital, Fudan University and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDeri
References
- Wang N, Fulcher J, Abeysuriya N, McGrady M, Wilcox I, Celermajer D, Lal S. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J 2019;40:476-84. [Crossref] [PubMed]
- Kim YJ, Kwon DA, Kim HK, Park JS, Hahn S, Kim KH, Kim KB, Sohn DW, Ahn H, Oh BH, Park YB. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009;120:1672-8. [Crossref] [PubMed]
- Taramasso M, Benfari G, van der Bijl P, Alessandrini H, Attinger-Toller A, Biasco L, et al. Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J Am Coll Cardiol 2019;74:2998-3008. [Crossref] [PubMed]
- Karam N, Braun D, Mehr M, Orban M, Stocker TJ, Deseive S, Orban M, Hagl C, Näbauer M, Massberg S, Hausleiter J. Impact of Transcatheter Tricuspid Valve Repair for Severe Tricuspid Regurgitation on Kidney and Liver Function. JACC Cardiovasc Interv 2019;12:1413-20. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs JESC/EACTS Scientific Document Group, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e72-e227. [Crossref] [PubMed]
- Dreyfus J, Dreyfus GD, Taramasso M. Tricuspid valve replacement: The old and the new. Prog Cardiovasc Dis 2022;72:102-13. [Crossref] [PubMed]
- Zhang Y, Lu F, Li W, Chen S, Li M, Zhang X, Pan C, Qiao F, Zhou D, Pan W, Ge J. A first-in-human study of transjugular transcatheter tricuspid valve replacement with the LuX-Valve Plus system. EuroIntervention 2023;18:e1088-9. [Crossref] [PubMed]
- Lu FL, Ma Y, An Z, Cai CL, Li BL, Song ZG, Han L, Wang J, Qiao F, Xu ZY. First-in-Man Experience of Transcatheter Tricuspid Valve Replacement With LuX-Valve in High-Risk Tricuspid Regurgitation Patients. JACC Cardiovasc Interv 2020;13:1614-6. [Crossref] [PubMed]
- Fam NP, von Bardeleben RS, Hensey M, Kodali SK, Smith RL, Hausleiter J, et al. Transfemoral Transcatheter Tricuspid Valve Replacement With the EVOQUE System: A Multicenter, Observational, First-in-Human Experience. JACC Cardiovasc Interv 2021;14:501-11. [Crossref] [PubMed]
- Navia JL, Kapadia S, Elgharably H, Harb SC, Krishnaswamy A, Unai S, Mick S, Rodriguez L, Hammer D, Gillinov AM, Svensson LG. First-in-Human Implantations of the NaviGate Bioprosthesis in a Severely Dilated Tricuspid Annulus and in a Failed Tricuspid Annuloplasty Ring. Circ Cardiovasc Interv 2017;10:e005840. [Crossref] [PubMed]
- Blanke P, Park JK, Grayburn P, Naoum C, Ong K, Kohli K, Norgaard BL, Webb JG, Popma J, Boshell D, Sorajja P, Muller D, Leipsic J. Left ventricular access point determination for a coaxial approach to the mitral annular landing zone in transcatheter mitral valve replacement. J Cardiovasc Comput Tomogr 2017;11:281-7. [Crossref] [PubMed]
- Ge Z, Pan C, Li W, Zhou D, Pan W, Wei L, Chen H, Shu X, Ge J. Real-Time Monitoring and Step-by-Step Guidance for Transapical Mitral Valve Edge-to-Edge Repair Using Transesophageal Echocardiography. J Interv Cardiol 2021;2021:6659261. [Crossref] [PubMed]
- Asmarats L, Dagenais F, Bédard E, Pasian S, Hahn RT, Navia JL, Rodés-Cabau J. Transcatheter Tricuspid Valve Replacement for Treating Severe Tricuspid Regurgitation: Initial Experience With the NaviGate Bioprosthesis. Can J Cardiol 2018;34:1370.e5-7. [Crossref] [PubMed]
- Webb JG, Chuang AM, Meier D, von Bardeleben RS, Kodali SK, Smith RL, et al. Transcatheter Tricuspid Valve Replacement With the EVOQUE System: 1-Year Outcomes of a Multicenter, First-in-Human Experience. JACC Cardiovasc Interv 2022;15:481-91. [Crossref] [PubMed]
- Liu Y, Chen B, Zhang Y, Zuo W, Li Q, Jin L, Kong D, Pan C, Dong L, Shu X, Ge J. Sources of Variability in Vena Contracta Area Measurement for Tricuspid Regurgitation Severity Grading: Comparison of Technical Settings and Vendors. J Am Soc Echocardiogr 2021;34:270-278.e1. [Crossref] [PubMed]
- Zoghbi WA, Asch FM, Bruce C, Gillam LD, Grayburn PA, Hahn RT, Inglessis I, Islam AM, Lerakis S, Little SH, Siegel RJ, Skubas N, Slesnick TC, Stewart WJ, Thavendiranathan P, Weissman NJ, Yasukochi S, Zimmerman KG. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2019;32:431-75. [Crossref] [PubMed]
- Tomlinson S, Rivas CG, Agarwal V, Lebehn M, Hahn RT. Multimodality imaging for transcatheter tricuspid valve repair and replacement. Front Cardiovasc Med 2023;10:1171968. [Crossref] [PubMed]
- Stankovic I, Daraban AM, Jasaityte R, Neskovic AN, Claus P, Voigt JU. Incremental value of the en face view of the tricuspid valve by two-dimensional and three-dimensional echocardiography for accurate identification of tricuspid valve leaflets. J Am Soc Echocardiogr 2014;27:376-84. [Crossref] [PubMed]
- Henry MP, Cotella JI, Slivnick JA, Yamat M, Hipke K, Johnson R, Mor-Avi V, Lang RM. Three-Dimensional Echocardiographic Deconstruction: Feasibility of Clinical Evaluation from Two-Dimensional Views Derived from a Three-Dimensional Data Set. J Am Soc Echocardiogr 2022;35:1009-1017.e2. [Crossref] [PubMed]
- Hahn RT, Saric M, Faletra FF, Garg R, Gillam LD, Horton K, Khalique OK, Little SH, Mackensen GB, Oh J, Quader N, Safi L, Scalia GM, Lang RM. Recommended Standards for the Performance of Transesophageal Echocardiographic Screening for Structural Heart Intervention: From the American Society of Echocardiography. J Am Soc Echocardiogr 2022;35:1-76. [Crossref] [PubMed]
- Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. [Crossref] [PubMed]


