
Cardiac magnetic resonance-guided recanalization for left anterior descending artery chronic total occlusion: a case description
Introduction
Despite the patency rate of chronic total occlusion (CTO) lesions being significantly improved due to technological advancements in percutaneous coronary intervention (PCI) (1), patient selection for CTO PCI should involve a consideration of myocardial viability to determine the potential clinical benefit (2). Here, we present a case treated with cardiac magnetic resonance (CMR)-guided PCI for left anterior descending artery (LAD) CTO with severe calcification.
Case presentation
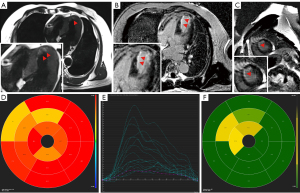
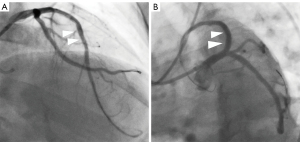
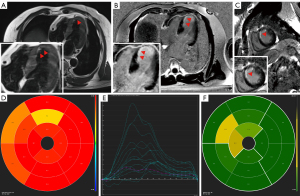
All procedures performed in this study were in accordance with the ethical standards of the relevant institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. In another hospital, eight months before attending our hospital, a 63-year-old male patient underwent diagnostic coronary angiography, which revealed severe stenosis in the left circumflex artery (LCX) and right coronary artery (RCA) as well as a CTO lesion in the LAD with grade 2 Werner collateral channels communicating to the LAD from the posterior branches of the left ventricle (LV). Two drug-eluting stents were implanted in the LCX (2.75 mm × 36 mm and 3.0 mm × 28 mm), and one was implanted in the RCA (3.5 mm × 36 mm) (Figure 1A-1C); meanwhile, conservative management was applied to the CTO. Transthoracic echocardiography (TTE) revealed an LV ejection fraction of 53% and an LV end-diastolic diameter of 63 mm with anterior wall myocardial thinning and motion hypokinesis. From then on, the patient received optimal drug treatment as recommended by the guidelines (3,4). However, due to recurrent symptom of chest distress lasting 2 months, he came to our hospital for further treatment. The admission examination showed a slight increase in the patient’s troponin level (0.10 ng/mL; reference value: 0–0.04 ng/mL). Additionally, the results of the treadmill exercise test were positive. We performed CMR examination on the patient to assess myocardial ischemia and viability in the CTO-subtended territory and determine the appropriate treatment approach, as a correlation between collateral flow and myocardial viability is lacking (5). CMR imaging showed subendocardial infarction in the anteroseptal and apical LV walls, with reduced myocardial strain in these segments (Figure 2A-2E). The extent of late gadolinium enhancement (LGE) was 20.4% (Figure 2F). According to previous study, the optimal cutoff value of LGE extent is less than 50% for detecting segments, indicating that the segments may functionally recover via CTO lesion revascularization (6). At the heart-team meeting, the patient was discussed in terms of symptoms (recurrent chest distress after optimal medical treatment) and angiography and CMR results (the extent of LGE was 20.4% which, being less than 50%, was predictive of functional recovery following revascularization of CTO), and PCI of the LAD-CTO with a primary antegrade strategy was scheduled. The treatment was performed via the left radial artery with a 7-Fr guiding catheter (LA6EBU375; Medtronic). A 6-Fr 3.5 Judkins right catheter was used for contralateral angiography. Initial attempts to deliver Fielder XT-R, Gaia first and Gaia second guidewires in sequence through the occluded segment of the LAD failed. Subsequently, a Gaia third wire was introduced and inserted successfully via antegrade wire escalation to open the occluded segment of the LAD. Three drug-eluting stents (3.0 mm × 36 mm, 3.0 mm × 36 mm, and 4.0 mm × 14 mm) were implanted in the LAD after rotational atherectomy with a 1.5 -mm burr at 170,000 rpm for 80 seconds, and thrombolysis in myocardial infarction (TIMI) III flow (normal flow with complete filling of the distal territory) was ensured after stent implantation (Figure 3). Four months after PCI, the patient’s symptoms markedly improved, and LV function slightly increased. CMR results showed significant improvement in myocardial strain in the anteroseptal and apical LV walls (Figure 4). The patient did not experience chest distress (New York Hear Association class I) during the 1-year clinical follow-up. In addition, the patient’s Seattle Angina Questionnaire results also showed significant improvement.

Discussion
PCI implemented via noninvasive imaging guidance, including echocardiography, computed tomography, nuclear scintigraphy, and CMR, has been reported to improve survival after revascularization in patients with coronary artery disease (7). CMR is a high-resolution imaging system that accurately evaluates cardiac dimensions and function and provides valuable information about myocardial viability with high sensitivity and specificity (6). In this case report, we describe a 63-year-old man undergoing PCI guided by CMR to address LAD-CTO.
Thus far, only a few randomized trials evaluating the safety and efficacy of CTO PCI have been conducted, with results being fairly equivocal. Moreover, the results of several observational studies appear inconsistent (8). In patients with multivessel coronary artery disease including a CTO, stenting obstructive non-CTO lesions while treating the CTO medically has been an alternative strategy that has been applied in real-world practice (9). However, whether all similar patients should undergo CTO PCI is unclear.
The relevant guidelines indicate that CTO PCI should be considered in patients who still have symptoms of angina pectoris after drug treatment is optimized or if a large-area ischemia has been confirmed in the territory of the occluded vessel (class recommendation: IIa; level of evidence: B) (10). Myocardial viability and ischemia are predictors of response to revascularization, and multiple noninvasive imaging modalities are available for evaluating them (11). CMR is a noninvasive, high-resolution imaging technique that evaluates the function and structure of the cardiovascular system. It uses multiparameter, multiplanar, and multisequence imaging to accurately display heart anatomical structures, such as myocardium, chambers, and valves; evaluate regional and global LV function; and detect the presence and the extent of myocardial infarction and ischemic burden. Compared with other noninvasive imaging techniques, CMR has the unique advantage simultaneously and directly imaging infarcted myocardium or scars with the normal myocardium (12). In a retrospective study of 59 patients, the functional improvement was significant after CTO PCI in the regions with a transmural LGE extent ≤50%, but there was no change in the region with LGE >50% (13). In our case, the patient experienced recurrent chest distress after optimal medical treatment, and there was clinical evidence of myocardial ischemia. Relieving symptoms and improving the patients’ quality of life remains the main indication for CTO PCI. Moreover, patients with ischemic cardiomyopathy who have viable myocardium may benefit from CTO PCI. CMR imaging can not only depict the structure and function of the heart but can also provide an evaluation of myocardial perfusion and myocardial injury patterns (14). Routine CMR is sufficient for evaluating myocardial viability, providing an important reference for CTO preoperative decision-making. In our case, CMR imaging importantly showed inducible ischemia in the territory of the LAD-CTO. Therefore, the anticipated benefits of the patient exceeded the potential risks which might have been caused by the procedure, and CTO-PCI was thus considered appropriate. Four months after PCI, CMR reexamination showed significant improvement in myocardial strain in the anteroseptal and apical LV walls. The patient had no further symptoms during 1 year of follow-up.
In conclusion, assessing myocardial viability and inducible perfusion defects in the CTO territory before PCI through CMR can help identify those patients who are more likely to benefit from CTO PCI. This case may serve as a reference for using CMR to evaluate and follow up patients with CTO lesions in clinical practice.
Conclusions
We report a case treated with CMR-guided PCI for LAD-CTO with severe calcification. The patient with three-vessel coronary artery disease was stented in obstructive non-CTO lesions while conservative management was applied for the CTO; subsequently, revascularization of the CTO under the guidance of CMR was performed after the angina was resistant to intensive medical therapy.
Acknowledgments
Part of the content in this article was published in the 34th Great Wall International Congress of Cardiology Asian Heart Society Congress 2023.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-894/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schumacher SP, Stuijfzand WJ, Opolski MP, van Rossum AC, Nap A, Knaapen P. Percutaneous Coronary Intervention of Chronic Total Occlusions: When and How to Treat. Cardiovasc Revasc Med 2019;20:513-22. [Crossref] [PubMed]
- Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019;140:420-33. [Crossref] [PubMed]
- Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2023;82:833-955. [Crossref] [PubMed]
- Section of Interventional Cardiology of Chinese Society of Cardiology, Section of Atherosclerosis and Coronary Artery Disease of Chinese Society of Cardiology, Specialty Committee on Prevention and Treatment of Thrombosis of Chinese College of Cardiovascular Physicians. Guideline on the diagnosis and treatment of stable coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi 2018;46:680-94. [Crossref] [PubMed]
- Wang L, Lu MJ, Feng L, Wang J, Fang W, He ZX, Dou KF, Zhao SH, Yang MF. Relationship of myocardial hibernation, scar, and angiographic collateral flow in ischemic cardiomyopathy with coronary chronic total occlusion. J Nucl Cardiol 2019;26:1720-30. [Crossref] [PubMed]
- Allahwala UK, Brilakis ES, Kiat H, Ayesa S, Nour D, Ward M, Lo S, Weaver JC, Bhindi R. The indications and utility of adjunctive imaging modalities for chronic total occlusion (CTO) intervention. J Nucl Cardiol 2021;28:2597-608. [Crossref] [PubMed]
- Patel P, Ivanov A, Ramasubbu K. Myocardial Viability and Revascularization: Current Understanding and Future Directions. Curr Atheroscler Rep 2016;18:32. [Crossref] [PubMed]
- Ybarra LF, Rinfret S, Brilakis ES, Karmpaliotis D, Azzalini L, Grantham JA, et al. Definitions and Clinical Trial Design Principles for Coronary Artery Chronic Total Occlusion Therapies: CTO-ARC Consensus Recommendations. Circulation 2021;143:479-500. [Crossref] [PubMed]
- Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, et al. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion. Circulation 2019;139:1674-83. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MOESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. Erratum in: Eur Heart J 2019;40:3096.
- Roifman I, Paul GA, Zia MI, Williams LK, Watkins S, Wijeysundera HC, Crean AM, Strauss BH, Dick AJ, Wright GA, Connelly KA. The effect of percutaneous coronary intervention of chronically totally occluded coronary arteries on left ventricular global and regional systolic function. Can J Cardiol 2013;29:1436-42. [Crossref] [PubMed]
- Al-Sabeq B, Nabi F, Shah DJ. Assessment of myocardial viability by cardiac MRI. Curr Opin Cardiol 2019;34:502-9. [Crossref] [PubMed]
- Nakachi T, Kato S, Kirigaya H, Iinuma N, Fukui K, Saito N, Iwasawa T, Kosuge M, Kimura K, Tamura K. Prediction of functional recovery after percutaneous coronary revascularization for chronic total occlusion using late gadolinium enhanced magnetic resonance imaging. J Cardiol 2017;69:836-42. [Crossref] [PubMed]
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720-826. [Crossref] [PubMed]


