
Disseminated peritoneal leiomyoma associated with pleural effusion: a case description and literature analysis
Introduction
Disseminated peritoneal leiomyomatosis (DPL), also known as leiomyomatosis peritonealis disseminata (LPD), is a rare benign disease affecting women of reproductive age that presents with multiple benign nodules scattered throughout the pelvis and peritoneal cavity. The disease lacks specific clinical manifestations and signs, and there are only about 200 cases in the literature. Since the popularization of laparoscopic techniques, the use of rotary cutters has been associated with an increased risk of secondary leiomyomas following laparoscopic surgery (1). Herein, we report a case of open myomectomy which developed into DPL 9 years after surgery in our hospital and was associated with pleural effusion.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. A 25-year-old woman, G0P0, was referred to hospital due to infertility and was diagnosed with a uterine leiomyoma (113 mm ´ 75 mm) on pelvic ultrasound. During an abdominal myomectomy performed in 2009, both ovaries appeared to be covered with small cysts, so an ovarian cyst excision was performed. Postoperative pathological examination revealed the following: uterine cellular leiomyoma and bilateral ovarian endometriosis. In 2014, she was admitted to the hospital because she was diagnosed with gestational hypertension-associated heart disease at this point. She was more than 5 months pregnant and underwent a caesarean section immediately. At that time, a pelvic ultrasound revealed a large myomatous mass, 111 mm ´ 91 mm in size, in the posterior part of the uterus. As the patient was unable to tolerate the operation, a hysteromyomectomy was not performed. In 2015, a pelvic ultrasound revealed a well-defined hysteromyoma measuring 79 mm ´ 60 mm, and open myomectomy was recommended because the patient wanted to prepare for pregnancy. Histological examination and immunohistochemistry (IHC) revealed a cellular uterine leiomyoma. Regular follow-up was then performed.
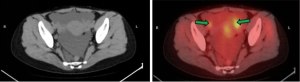
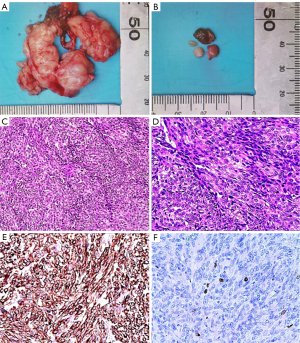
The patient was admitted to the hospital in July 2017 due to having suddenly developed abdominal pain and bloating. A pelvic ultrasound scan showed multiple irregular pelvic masses with a dominant mass measuring 27 mm in diameter. Positron emission tomography-computed tomography (PET-CT) showed multiple tumors attached to the peritoneum and bowel, as well as ascites (Figure 1). Cancer antigen 125 (CA-125) was elevated at 612 U/mL, but other tumor markers were within normal limits: carcinoembryonic antigen (CEA) 1.06 ng/mL; human epididymal protein 4 (HE4) 51.8 pmol/L; alpha fetoprotein (AFP) 3.25 IU/mL. There was a high suspicion of malignant tumor metastasis and a diagnostic laparoscopy was performed. Numerous nodules ranging from 1 to 40 mm in diameter were identified in the omentum, peritoneum, pouch of Douglas, serosal surface of the colon, and rectum. Frozen section pathology at surgery revealed a benign tumor. As the patient was planning to have children, she subsequently underwent laparoscopic excision of some of the disseminated nodules. A sample of ascitic fluid was taken for examination. Pathological examination of the specimens was performed after surgery. Gross findings (Figure 2A,2B) showed that the masses consisted of grey-white nodules with intact surface capsules. Microscopy (Figure 2C,2D) revealed a fusiform structure. This consisted of swirling smooth muscle cells. The spindle cells were uniform in size and showed no atypia, nuclear division, or invasive growth pattern. IHC staining showed that the spindle cells were positive for H-caldesmon (Figure 2E), desmin, smooth muscle actin (SMA), estrogen receptor (ER), and progesterone receptor (PR); the Ki-67 index (Figure 2F) was <10%, pan-cytokeratin (CK), CD34, CD99, CD117, S100, HMB45, and DOG-1 staining were negative. There was no cytological evidence of malignancy after ascitic fluid samples. The diagnosis was DPL. No intraoperative or postoperative complications were reported, and the patient was discharged 7 days after surgery.
In July 2018, the patient experienced chest tightness, progressive shortness of breath, and abdominal distension. Her CA-125 level was measured at 612 U/mL. Chest CT showed bilateral pleural effusion and no pleural nodules (Figure 3A,3B). The specific gravity of pleural effusion was 1.018, lactate dehydrogenase (LDH) was 119 U/L, albumin was 31.3 g/L, Rivalta test was positive, and pleural effusion LDH/plasma LDH >0.6. The fluid was a transudate. Pelvic ultrasound detected multiple solid masses in the pelvis and abdomen, with a maximum diameter of 4.47 cm. There was no cytological evidence of malignancy after examination of pleural effusion samples. The patient had an excellent response to subcutaneous leuproline and oral letrozole, with relief of pleural effusion and significant nodule shrinkage.
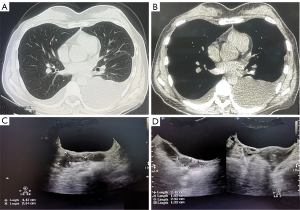
In January 2019, at regular follow-up, the patient remained asymptomatic and pelvic ultrasound also confirmed a continuous shrinkage of the largest nodule. The largest nodule showed a 30% decrease in the sum of the longest diameters from pretreatment (July 2018) (Figure 3C) to posttreatment (January 2019) (Figure 3D).
As the patient was infertile, it was recommended that the frozen embryo be thawed and transferred in the future, and that bilateral salpingectomy and oophorectomy be performed as a priority. The patient opted for assisted reproductive treatment at another hospital after a 6-month follow-up.
Discussion
The etiology of LPD remains controversial. Currently, the peritoneal metaplasia hypothesis, hormonal factors, iatrogenic factors, and genetic factors are thought to be associated with the occurrence of LPD (2). It is now generally accepted that estrogen plays an important role in the onset and development of LPD (3). The reports of LPD cases during pregnancy, hormone replacement therapy, and tamoxifen therapy support this hypothesis. As the hormone levels in the body decrease, the LPD nodules may shrink or disappear. Less than 5 years after surgery, the patient became pregnant and had a caesarean section. Hormonal stimulation can also be an important cause of DPL.
In recent years, cases of LPD following laparoscopic uterine surgery have been frequently reported (4). In 2014, an alarm was sounded for the control of iatrogenic LPD when the Food and Drug Administration (FDA) issued a consensus on the use of laparoscopic uterine electric morcellators (5). This patient had no previous laparoscopic surgery and no family history, which provides a new idea for the etiological study of LPD.
LPD has been reported to be associated with endometriosis. Toriyama et al. (6) reported that both endometriosis and LPD are derived from mesenchymal stem cells. Peritoneal mesenchymal stem cells can be converted not only into smooth muscle cells, but also into endometrial glands and stroma. The use of aromatase inhibitors (7) or gonadotropin hormone-releasing hormone (GnRHa) (8) to reduce estrogen can inhibit the growth of peritoneal leiomyoma nodules. In this case, ovarian endometriosis was found at the first operation. When aromatase inhibitor and GnRHa were used in the later treatment, the patient’s symptoms improved, further suggesting that there is a certain correlation between LPD and endometriosis. However, further research is needed to determine whether LPD nodules originate from ectopic endometrium or from different metaplasias of the same tissue.
Some LPD patients are asymptomatic, whereas others may present with a variety of symptoms, including abdominal pain and pelvic compression (9). Diagnosis is difficult because of these non-specific symptoms, and pelvic ultrasound and magnetic resonance imaging (MRI) show scattered pelvic and abdominal nodules of different sizes. On PET, some researchers have reported that the nodules show avid fluorodeoxyglucose (FDG) uptake (10), whereas others have shown low 18F-FDG avidity with the highest measured at maximum standardized uptake value (SUVmax) 2.9 (11). The tumors seen in this case of LPD where PET-CT was performed had low 18F-FDG avidity.
To date, no specific serological markers for LPD have been identified. A small number of patients with LPD have elevated serum CA125 (12), but the reason for these elevations is unclear. Therefore, LPD is most commonly diagnosed by histopathological examination and IHC (13). In this case, IHC staining showed typical smooth muscle tumors expressing H-caldesmon, vimentin, desmin, and SMA. Gross pathology of the patient showing H-caldesmon (+) and desmin (+).
There is no uniform diagnosis and treatment plan for LPD worldwide. At present, it is believed that the treatment of LPD should follow the principle of individualized treatment, mainly surgical treatment, and choose different treatment schemes according to the patient’s age, fertility requirements, and lesion size. In patients without fertility needs, the entire uterus, bilateral adhesions, and omentum should be removed, and pelvic and abdominal lesions should be removed as much as possible, to reduce the malignant transformation rate and recurrence rate. If laparoscopic surgery is chosen, the use of rotary cutters should be avoided as far as possible.
As LPD is mostly benign and closely related to hormone levels in the body, hormone withdrawal treatment can reduce or even eliminate the lesions. Patients who have not yet given birth should have as many lesions removed as possible to preserve their reproductive function (14), stop taking oral contraceptives, and avoid sexual hormone stimulation. After surgery, GnRHa, aromatase inhibitors, estrogen inhibitors, and other drugs may be used to reduce the level of the sex hormone in the body. In this case of pleural effusion, malignant pleural effusion could be excluded by IHC and cytomorphology examination of the pleural effusion. The main treatment is the drainage of the pleural effusion. At the same time, GnRHa and aromatase inhibitors can significantly reduce pleural effusion.
Acknowledgments
We acknowledge the patient who gave her consent for the publication of this article.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-398/coif). The authors report that this work was supported by Gansu Province Science Foundation for Youths (No. 21JR1RA006), the Innovation and Entrepreneurship Talent Project of Lanzhou (No. 2020-RC-52), and Natural Science Foundation of Gansu Province of China (Grant No. 21JR1RA012). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alonso-Pacheco L, Rodrigo-Olmedo M. Leiomyomatosis peritonealis disseminate. Laparoscopic management. Ginecol Obstet Mex 2014;82:350-3.
- Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest 2010;69:239-44. [Crossref] [PubMed]
- Nassif GB, Galdon MG, Liberale G. Leiomyomatosis peritonealis disseminata: case report and review of the literature. Acta Chir Belg 2016;116:193-6. [Crossref] [PubMed]
- Van der Meulen JF, Pijnenborg JM, Boomsma CM, Verberg MF, Geomini PM, Bongers MY. Parasitic myoma after laparoscopic morcellation: a systematic review of the literature. BJOG 2016;123:69-75. [Crossref] [PubMed]
- Belmarez JA, Latifi HR, Zhang W, Matthews CM. Simultaneously occurring disseminated peritoneal leiomyomatosis and multiple extrauterine adenomyomas following hysterectomy. Proc (Bayl Univ Med Cent) 2019;32:126-8. [Crossref] [PubMed]
- Toriyama A, Ishida M, Amano T, Nakagawa T, Kaku S, Iwai M, Yoshida K, Kagotani A, Takahashi K, Murakami T, Okabe H. Leiomyomatosis peritonealis disseminata coexisting with endometriosis within the same lesions: a case report with review of the literature. Int J Clin Exp Pathol 2013;6:2949-54.
- Takeda T, Masuhara K, Kamiura S. Successful management of a leiomyomatosis peritonealis disseminata with an aromatase inhibitor. Obstet Gynecol 2008;112:491-3. [Crossref] [PubMed]
- Hales HA, Peterson CM, Jones KP, Quinn JD. Leiomyomatosis peritonealis disseminata treated with a gonadotropin-releasing hormone agonist. A case report. Am J Obstet Gynecol 1992;167:515-6. [Crossref] [PubMed]
- Declas E, Lucot JP. Extra uterine leiomyomatosis: Review of the literature. Gynecol Obstet Fertil Senol 2019;47:582-90. [Crossref] [PubMed]
- Rodríguez García P, Castañer Ramón-Llín J, Romera Barba E, Sánchez Pérez A, Vázquez Rojas JL. Disseminated peritoneal leiomyomatosis, a diagnostic challenge. Gastroenterol Hepatol 2019;42:554-5. [Crossref] [PubMed]
- Khoo ACH, Lim SY. (18)F-fluorodeoxyglucose positron emission tomography-computed tomography imaging of leiomyomatosis peritonealis disseminata. World J Nucl Med 2021;20:322-3. [Crossref] [PubMed]
- Benlolo S, Papillon-Smith J, Murji A. Ulipristal Acetate for Disseminated Peritoneal Leiomyomatosis. Obstet Gynecol 2019;133:434-6. [Crossref] [PubMed]
- Halama N, Grauling-Halama SA, Daboul I. Familial clustering of Leiomyomatosis peritonealis disseminata: an unknown genetic syndrome? BMC Gastroenterol 2005;5:33. [Crossref] [PubMed]
- Lee WY, Noh JH. Leiomyomatosis peritonealis disseminata associated with appendiceal endometriosis: a case report. J Med Case Rep. 2015;9:167. [Crossref] [PubMed]


