
Clinical application of intravascular forceps biopsy in the diagnosis of vascular obstructive diseases: a pilot study
Introduction
Vascular obstruction disease (VOD) may be caused by malignant tumors or benign diseases. The former mainly involves malignancies with metastasis, infiltration, or primary vascular tumors, and the latter mainly involves blood thrombus, reactive changes, or benign tumors (1). Different etiologies imply different pathologic features, prognoses, and treatment strategies. Hence, early and precise diagnosis is essential to formulating individualized precise treatment strategies during clinical practice. Modern medical imaging techniques, such as ultrasound (US), computed tomography (CT), positron emission tomography-CT (PET-CT), and magnetic resonance imaging (MRI) (2-5), can distinguish malignancies and benign diseases to some extent. Given that histopathology remains the gold standard for final diagnosis, it is critical to obtain high quality tissue samples.
Unfortunately, when a suspected mass is detected within the blood vessel, the options for effective tissue sampling are severely limited. The conventional sampling method is percutaneous needle biopsy, which is generally only recommended for patients with extensive intravascular lesions, as puncture damage to the vascular wall may lead to significant bleeding or implanted metastasis. Moreover, the technical success rate may decrease due to limited sampling arising from concerns of bleeding (6).
Based on our previous experiences with percutaneous transhepatic biliary forceps biopsies (7,8), intravascular forceps biopsy (IVFB) may be an effective alternative method for VOD, a component of planned revascularization procedures through an established vascular access, or an alternative when percutaneous needle biopsies fail or no safe puncture needle access can be chosen. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-597/rc).
Methods
Patients
All data in this retrospective study were collected from the First Affiliated Hospital of Zhengzhou University electronic information system. From January 2015 to January 2022, 35 nonconsecutive patients (21 men and 14 women) with a mean age of 60±11 years (range, 39–81 years) underwent fluoroscopy-guided IVFB for VOD at our department. The detailed information on pre-, intra- and post-IVFB is listed in Table 1. The inclusion criteria were the following: (I) an age from 18 to 85 years; (II) preoperative imaging showing obvious intravascular mass or severe stenosis disease (vascular stenosis above 50%), (III) failure of percutaneous needle biopsy, and (IV) no safe percutaneous needle puncture access. The exclusion criteria were (I) platelet count ≤30×109/L or prothrombin time >21 s and (II) New York Heart Association class III–IV. The study workflow is presented in Figure 1.
Table 1
| Case | Gender/age (years) | Main symptoms | History of malignancy | Location | Indication for IVFB | Final histopathology | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| 1 | M/45 | Head and neck swelling | NSCLC | SVC | PRP | NSCLC | 8.6 |
| 2 | F/56 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 12.4 |
| 3 | M/55 | Ascites, abdominal pain | HCC | PV | PRP | HCC | 11.5 |
| 4 | M/46 | Head and neck swelling | NSCLC | SVC | PRP | NSCLC | 16.1 |
| 5 | F/72 | Intermittent dyspnea | No | LPA | Puncture biopsy failure | Angiosarcoma | 13.2 |
| 6 | F/55 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 23.8 |
| 7 | M/45 | Lower limb swelling | Clear-cell carcinoma of right kidney | IVC | PRP | Clear cell carcinoma | 10.9 |
| 8 | M/48 | Head and neck swelling | Non-Hodgkin lymphoma | SVC | PRP | Non-Hodgkin lymphoma | 28.4 |
| 9 | M/65 | Portal hypertension, gastrointestinal bleeding | HCC | PV | PRP | HCC | 12.0 |
| 10 | M/81 | Left lower limb swelling | No | LCIV | PRP | Blood thrombus | 17.5 |
| 11 | F/71 | Right lower limb swelling | Non-Hodgkin lymphoma | RCIV | PRP | Blood thrombus | 17.3 |
| 12 | F/52 | Ascites, abdominal pain | HCC | PV | PRP | HCC | 11.0 |
| 13 | F/56 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 31.6 |
| 14 | M/59 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 17.2 |
| 15 | M/61 | Abdominal pain | HCC | PV | PRP | HCC and blood thrombus | 9.3 |
| 16 | F/62 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 17.8 |
| 17 | F/63 | Head and neck swelling | Esophageal carcinoma | SVC | PRP | Blood thrombus | 20.6 |
| 18 | M/78 | Chest pain, Intermittent dyspnea | No | RPA | Percutaneous biopsy failure | Angiosarcoma | 7.7 |
| 19 | M/46 | Ascites | HCC | PV | PRP | HCC | 13.4 |
| 20 | F/56 | Ascites, abdominal pain | HCC | PV | PRP | HCC | 15.1 |
| 21 | F/39 | Lower limb swelling | Clear cell carcinoma of right kidney | IVC | PRP | Clear cell carcinoma | 24.5 |
| 22 | M/67 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 33.7 |
| 23 | F/61 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 21.5 |
| 24 | M/80 | Head and neck swelling | NSCLC | SVC | PRP | NSCLC | 9.8 |
| 25 | F/73 | Chest pain | No | MPA | PRP | Blood thrombus | 22.3 |
| 26 | M/75 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 17.4 |
| 27 | M/64 | Chest pain, Intermittent dyspnea | Lung squamous cell carcinoma | MPA | No safe puncture access | Lung squamous cell carcinoma | 9.2 |
| 28 | M/45 | Abdominal pain | HCC | PV | PRP | HCC | 11.7 |
| 29 | M/49 | Head and neck swelling | NSCLC | SVC | PRP | NSCLC | 15.1 |
| 30 | M/51 | Head and neck swelling | NSCLC | SVC | PRP | NSCLC | 19.3 |
| 31 | F/55 | Extremity swelling | No | IVC | PRP | Blood thrombus | 26.8 |
| 32 | M/61 | Head and neck swelling | Lung squamous cell carcinoma | SVC | PRP | Lung squamous cell carcinoma | 19.5 |
| 33 | M/68 | Ascites, abdominal pain | HCC | PV | PRP | HCC | 11.3 |
| 34 | F/72 | Lower limb swelling | No | IVC | PRP | Blood thrombus | 33.8 |
| 35 | M/74 | Head and neck swelling | Lung squamous cell carcinoma | SVC | PRP | Lung squamous cell carcinoma | 13.6 |
IVFB, intravascular forceps biopsy; M, male; F, female; NSCLC, non-small cell lung cancer; SVC, superior vena cava; IVC, inferior vena cava; HCC, hepatocellular carcinoma; PV, portal vein; PRP, planned revascularization procedure; LPA, left pulmonary artery; LCIV, left common iliac vein; RCIV, right common iliac vein; RPA, right pulmonary artery; MPA, main pulmonary artery.
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University (ethical review No. 2022-KY-200). The requirement for informed consent was waived due to the retrospective nature of the study.
Procedures
Femoral vein (FV) access can be used for superior vena cava (SVC), inferior vena cava (IVC), and pulmonary artery (PA) biopsies, while percutaneous transhepatic puncture access can be used for portal vein (PV) biopsies. The procedure is demonstrated in Figures 2-5.
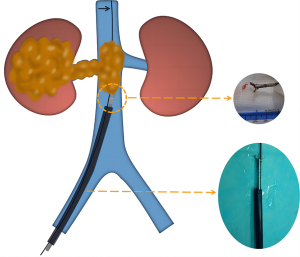


IVFB for the SVC, IVC, and PA
Sedation was achieved by continuous infusion of propofol (4–6 mg/kg, Jiangsu Enhua Pharmaceutical Co., Ltd., Xuzhou, China). Local anesthesia with 2% lidocaine (5–10 mL) was applied to the puncture site. The steps for the procedure were as follows: (I) the FV access was successfully established by a 9F sheath (90 cm in length for the SVC and PA; 45 cm for the IVC; Cook Medical, Indiana, USA) under the guidance of digital subtraction angiography (DSA; Artis-zeego, Siemens Healthineers, Munich, Germany). (II) A 0.035-inch soft guide wire (Terumo Medical, Tokyo, Japan) and a 5F catheter (100 cm in length; Cook Medical) were introduced through the sheath and reopened the obstructive blood vessels. (III) The initial soft guide wire was replaced with strengthened guide wire to adjust the sheath direction. (IV) The 9F sheath was advanced to the proximal end of the vascular obstructive site, and the location was confirmed by vascular angiography. (V) Forceps biopsy (clamp diameter =6 mm, length =120 mm; Nanjing Micro-tech) was introduced through the 9F sheath to the obstructed area, pushed forward 5–10 mm, and then tightened and pulled out of the sheath. The specimens were evaluated by expert pathologists. The IVFB was performed 3–6 times to obtain satisfactory specimens.
IVFB for the PV
The anesthetic method of IVFB for the PV was the same as that described for that of the SVC, IVC, and PA. The steps for the procedure were as follows: (I) the PV was successfully punctured by a 21 G × 15 cm Chiba needle (Cook Medical) under the guidance of US (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) and DSA. (II) A 0.018 inch × 30 cm platinum micro-guide wire was introduced and then replaced with a 6 F × 20 cm expander (Cook Medical). (III) The 9 F sheath (23 cm in length; Cordis, Florida, USA) was delivered into the vascular obstruction position with the tip above the PV obstructive site. (IV) IVFB was completed through the sheath 3–6 times in the same manner as that described for the SVC, IVC, and PA.
Definition and follow-up
Technical success was defined as successful tissue specimen sampling according to the pathologist’s confirmation. The final malignancy diagnosis in each patient was dependent on the surgical pathological results, IVFB, other malignant cytological evidence (i.e., cancer cells found in pleural and peritoneal effusion), or clinical imaging follow-up within 6 months (clinical diagnosis was established as malignant if progressive disease, including the enlargement of the lesion, and a new lesion or metastasis of the lymph node or other organs, were observed). Sensitivity was defined as the number of people with both IVFB and a final diagnosis of malignancy divided by the number of people with a final diagnosis of malignancy. Specificity was defined as the number of people with both IVFB and a final diagnosis of benign disease divided by the number of people with a final diagnosis of benign disease. Positive predictive value (PPV) and negative predictive value (NPV) were calculated based on sensitivity and specificity. Accuracy rate (AR) was defined as the number of IVFB diagnoses that were consistent with the final diagnosis divided by the total number of people. Complications were reported according to the Society of Interventional Radiology (SIR) Standards of European Radiological Practice Committee classification (9).
Statistical analyses
SPSS software (IBM Corp., New York, USA) was used to perform all statistical analyses. Continuous variables are presented as the mean ± standard deviation (normally distributed variables) or median with range (nonnormalized variables); categorical variables are presented as counts and percentages. Sensitivity, specificity, PPV, NPV, and accuracy are provided as measures of diagnostic performance. A two-tailed P value <0.05 was considered significantly different.
Results
A total of 35 patients (21 males, 14 females; mean age 60±11 years; range, 39–81 years) underwent IVFB: 32 (91.4%) during interventional planned revascularization procedures and 3 (8.6%) due to inaccessible or failed percutaneous needle access. The technical success was 100%. The mean procedure time, median number of biopsies taken per biopsy session, and median patient radiation dose (PRD) were 30.9±6.9 min, 4 (range, 3–6), and 712.6 mGy (range, 383.4–1,450.8 mGy), respectively. More detailed information is list in Table 1.
Among 35 biopsy procedures, the diagnosis was malignant disease in 21 patients (60.0%), with histopathological results indicating hepatocellular carcinoma (n=7), hepatocellular carcinoma with thrombosis (n=1), non-small cell lung cancer (n=5), lung squamous cell carcinoma (n=3), angiosarcoma (n=2), clear cell carcinoma of kidney (n=2), or non-Hodgkin lymphoma (n=1). The diagnosis for the 14 remaining patients was benign disease. However, three patients (21.4%) diagnosed with benign disease were considered false negatives. Further follow-up imaging revealed evidence of malignancy in these patients, two of whom were finally diagnosed as non-Hodgkin lymphoma and esophageal cancer, which was consistent with a malignant history; the other patient had no malignant history and was finally diagnosed with primary angiosarcoma in the second IVFB. In summary, the overall sensitivity, specificity, PPV, NPV, and AR of IVFB were 87.5% (21/24), 100% (11/11), 100% (21/21), 78.6% (11/14), and 91.4% (32/35), respectively. There were no complications related to IVFB such as hemorrhage or perforation.
Discussion
The etiology of VOD is complicated, including benign and malignant tumors, thrombus, etc. Different types of VOD require different treatment options naturally lead to different clinical outcomes. For example, PA sarcoma (PAS) is the most well-known primary vascular malignancy, with a median survival of less than 1 year for patients with incomplete resection (10). Furthermore, there are various subtypes of PA sarcomas, such as rhabdomyosarcoma, osteogenic sarcoma, angiosarcoma, fibrosarcoma, myxosarcoma, and liposarcoma, and the distinction between the different subtypes requires histological assessment (11). Although US, CT, MRI, and PET-CT can help differentiate tumors and blood thrombus to a certain extent, with high sensitivity and specificity [92% and 92% for US (12), 92% and 86% for CT (3), 98% and 68% for MRI (5), and 71.4% and 90% for PET-CT, respectively (13)], which can identify the vascularity of intravascular masses, judge the degree of obstruction, and demonstrate the relationship between masses and the involved vessels, such as infiltration or adherence to the vascular wall. However, they still cannot provide pathological diagnosis.
Percutaneous needle biopsy is a relatively less invasive intervention than is surgery for examining solid endovascular lesion (14,15), but it has its own disadvantages: (I) in the case of deep veins such as the PV and SVC, inevitably passing through vital organs and tissues increases the risk of injury and bleeding; (II) a large amount of collateral circulation develops around the obstructed vessels, and direct puncture of high-pressure vessels or the surrounding collateral circulation can lead to major bleeding events (16); and (III) there is also a theoretical occurrence of implanted metastasis (17).
In contrast to the percutaneous needle biopsy, IVFB does not puncture the obstructive area of blood vessels directly, which is theoretically safer and has a lower risk of bleeding. Robins first reported a case in 1972, when IVFB was used to diagnose Budd-Chiari syndrome secondary to real carcinoma invading the IVC (18). Subsequently, Withers reported four cases (three with IVC and one with suspected iliac vein malignant tumors) using IVFB (19). Sherk et al. reported the largest sample of IVFB in 2019, which showed that 36 patients underwent IVFB for suspected tumor thrombus or perivascular tumor with 100% technical success and 0% complications, with 75% of the IVFBs being performed during the planned revascularization procedures (20).
This pilot study showed that the technical success and AR of IVFB were 100% and 91.4%, respectively, without related complications, which suggests it is a feasible and safe sampling method. The core technological advantage of IVFB was that the strengthened guide wire was used to determine the direction of the sheath, which was targeted at the obstruction site, and the remaining space in the sheath was provided for the delivery of the biopsy forceps to sample the vascular mass. IVFB can be considered to be an alternative when imaging findings are ambiguous or when the patient is not eligible for percutaneous biopsy. When patients are scheduled to undergo revascularization procedures, IVFB can be used as a concurrent method to provide patients with direct pathological evidence.
In regards to improving the sensitivity of IVFB, our experiences were the following: (I) IVFB should be performed before balloon dilatation or stenting, and theoretically, forceps biopsy can be more efficient at narrow lumens. (II) IVFB is recommended to be performed at the narrowest part because it is the most typical representative of histology based on angiography and sampling position; however, this is only speculative, and more clinical data are needed to confirm this. (III) Three to six biopsies can be completed to obtain a sufficient amount of tissue according to operator’s experience. (IV) It is recommended that pathologists evaluate the samples on site to determine the quality of specimens. (V) After the specimen is fixed, it should be immediately sent to the pathology department to prevent the tissue from being dehydrated according to the recommendations of pathologists. (VI) Proficient catheter guide wire manipulation is also crucial for improving technical success rates; for example, correct position of the sheath tip is necessary for accurate biopsy and particularly important for PA endovascular biopsy due to its long and curved path.
It is worth noting false negatives were produced in three patients in this study, indicating that this technology also has certain limitations. Under angiography, the narrowing of vessels indirectly indicates the degree of tumor invasion. The narrower the vessels are, the more severe the tumor invasion, and IVFB is more capable of obtaining representative samples. However, when the degree of vascular stenosis is mild, the accuracy of IVFB will theoretically decrease to some extent. Analyzing these three patients, we found that the degree of vascular stenosis in these three cases were 59%, 61% and 70%, respectively, while in the other patients, the degree of vascular stenosis was over 75%. In the future, by increasing the sample size, multiple factor analysis can be conducted on the degree of vascular stenosis and positivity rate.
Our study has some limitations. First, we employed a retrospective design with a small sample size, and the conclusion needs to be confirmed by prospective, multiple-center, large-sample studies in the future. Moreover, some tissues may be more easily biopsied than others (e.g., fresh thrombus vs. partially calcified mesenchymal tumors), and there is a possibility that the surrounding thrombus can be biopsied, while the more deeply embedded or distal tumors can be missed. Another technical limitation of the biopsy instrument is that it probably best samples tissue directly ahead of it, and sampling tissue on the side of the lumen would involve trying to angle the biopsy forceps toward a perpendicular angle, which may not be possible in certain blood vessels due to the vessel size.
Conclusions
Our study suggests that IVFB is a safe and accurate procedure for tissue sampling for suspected intravascular masses and can be conveniently performed during vascular interventional procedures.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-597/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-597/coif). Mengyao Song, X.Z., X.H., and D.J. report funding support from the Henan Province Science and Technology Research Project (grant No. 232102311132). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University (ethical review No. 2022-KY-200). The requirement for informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pomoni A, Sotiriadis C, Gay F, Jouannic AM, Qanadli SD. Percutaneous endovascular biopsy of intravascular masses: efficacy and safety in establishing pre-therapy diagnosis. Eur Radiol 2018;28:301-7. [Crossref] [PubMed]
- Tarantino L, Francica G, Sordelli I, Esposito F, Giorgio A, Sorrentino P, de Stefano G, Di Sarno A, Ferraioli G, Sperlongano P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging 2006;31:537-44. [Crossref] [PubMed]
- Ascenti G, Sofia C, Mazziotti S, Silipigni S, D'Angelo T, Pergolizzi S, Scribano E. Dual-energy CT with iodine quantification in distinguishing between bland and neoplastic portal vein thrombosis in patients with hepatocellular carcinoma. Clin Radiol 2016;71:938.e1-9. [Crossref] [PubMed]
- Wu B, Zhang Y, Tan H, Shi H. Value of (18)F-FDG PET/CT in the diagnosis of portal vein tumor thrombus in patients with hepatocellular carcinoma. Abdom Radiol (NY) 2019;44:2430-5. [Crossref] [PubMed]
- Abd El Salam fayed AA, Sami HA, Kamel AH, El Baz TM, Abd El Fattah Hassan Gadalla A, Mahmoud BE. Discrimination between malignant and bland portal vein thrombosis: could diffusion weighted MRI help. The Egyptian Journal of Radiology and Nuclear Medicine 2018;49:608-13.
- Sainani NI, Arellano RS, Shyn PB, Gervais DA, Mueller PR, Silverman SG. The challenging image-guided abdominal mass biopsy: established and emerging techniques 'if you can see it, you can biopsy it'. Abdom Imaging 2013;38:672-96. [Crossref] [PubMed]
- Wu ZY, Jiao DC, Guo FF, Zhang DD, Ren JZ, Han XW. Treatment of biliary stenosis using percutaneous transhepatic cholangiobiopsy with biopsy forceps of varying diameter. Quant Imaging Med Surg 2022;12:207-14. [Crossref] [PubMed]
- Liu Y, Zhou X, Kong L, Han X, Jiao D. Percutaneous transhepatic intraluminal forceps biopsy for patients with biliary stricture after endoscopic retrograde approach failure: a retrospective study. Quant Imaging Med Surg 2023;13:2605-19. [Crossref] [PubMed]
- Cardella JF, Kundu S, Miller DL, Millward SF, Sacks DSociety of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2009;20:S189-91. [Crossref] [PubMed]
- Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR, Walkes JC, Walsh GL, Moran C, Singh H, Vaporciyan AA, Reardon M. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009;87:977-84. [Crossref] [PubMed]
- Nonomura A, Kurumaya H, Kono N, Nakanuma Y, Ohta G, Terahata S, Matsubara F, Matsuda T, Asaka T, Nishino T. Primary pulmonary artery sarcoma. Report of two autopsy cases studied by immunohistochemistry and electron microscopy, and review of 110 cases reported in the literature. Acta Pathol Jpn 1988;38:883-96. [Crossref] [PubMed]
- Sorrentino P, Tarantino L, D'Angelo S, Terracciano L, Ferbo U, Bracigliano A, Panico L, De Chiara G, Lepore M, De Stefano N, Fiorentino F, Vecchione R. Validation of an extension of the international non-invasive criteria for the diagnosis of hepatocellular carcinoma to the characterization of macroscopic portal vein thrombosis. J Gastroenterol Hepatol 2011;26:669-77. [Crossref] [PubMed]
- Sharma P, Kumar R, Jeph S, Karunanithi S, Naswa N, Gupta A, Malhotra A. 18F-FDG PET-CT in the diagnosis of tumor thrombus: can it be differentiated from benign thrombus? Nucl Med Commun 2011;32:782-8. [Crossref] [PubMed]
- Rammohan A, Jeswanth S, Sukumar R, Anand L, Kumar PS, Srinivasan UP, Ravi R, Ravichandran P. Percutaneous ultrasound-guided fine-needle aspiration of portal vein thrombi as a diagnostic and staging technique for hepatocellular carcinoma. Abdom Imaging 2013;38:1057-60. [Crossref] [PubMed]
- Dodd GD 3rd, Carr BI. Percutaneous biopsy of portal vein thrombus: a new staging technique for hepatocellular carcinoma. AJR Am J Roentgenol 1993;161:229-33. [Crossref] [PubMed]
- Montani D, Jaïs X, Sitbon O, Dartevelle P, Simonneau G, Humbert M. EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. Eur Respir J 2012;39:1549-50. [Crossref] [PubMed]
- Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Radiology 1991;178:253-8. Review. [Crossref] [PubMed]
- Robins JM, Bookstein JJ. Percutaneous transcaval biopsy technique in the evaluation of inferior vena cava occlusion. Radiology 1972;105:451-2. [Crossref] [PubMed]
- Withers CE, Casola G, Herba MJ, Viloria J. Intravascular tumors: transvenous biopsy. Radiology 1988;167:713-5. [Crossref] [PubMed]
- Sherk WM, Khaja MS, Majdalany BS, Saad WE, Udager AM, Cooper KJ, Williams DM. Transvenous Biopsy in the Diagnosis of Intravascular or Perivascular Neoplasm: A Single-Center Retrospective Analysis of 36 Patients. J Vasc Interv Radiol 2019;30:54-60. [Crossref] [PubMed]



