
Impact of deep learning-based reconstruction and anti-peristaltic agent on the image quality and diagnostic performance of magnetic resonance enterography comparing single breath-hold single-shot fast spin echo with and without anti-peristaltic agent
Introduction
For high-quality magnetic resonance enterography (MRE), adequate bowel distension and the absence of bowel movement are essential (1). Anti-peristaltic agents are widely used to decrease bowel peristalsis and improve bowel distension, subsequently reducing image blurring, which could degrade image quality. Although a previous study reported that diagnostic confidence remains high without using an anti-peristaltic agent (2), its administration is considered beneficial for enhancing image quality, and as a result, current international guidelines suggest considering its application (3,4).
Although the time and dose of administration vary among institutions, spilt-dose administration is considered more effective than single-dose administration for reducing peristalsis (5). Current guidelines recommend split-dose administration for improving image quality, and many institutions follow this recommendation (6,7). T1-weighted three-dimensional (3D) spoiled gradient-echo sequences are most susceptible to bowel movement; therefore, injection of anti-peristaltic agents before T1-weighted imaging (T1WI) is a common practice (1). Moreover, a previous study demonstrated that omitting anti-peristaltic agent administration could decrease the sensitivity of diffusion-weighted imaging (DWI) for diagnosing bowel inflammation in Crohn’s disease (8). Thus, anti-peristaltic agents are administered before T2WI, DWI, and T1WI at our institution.
Despite the benefits of anti-peristaltic agents, their use could be limited owing to the side effects attributed to their anticholinergic properties, and increased complexity and expense of the examination. Therefore, reducing the use of anti-peristaltic agents, while preserving the diagnostic performance could make MRE examinations more convenient, less costly, and less likely to result in adverse effects.
Single-shot fast spin-echo (SSFSE), which obtains images within a single echo train, is routinely used for T2-weighted MRE. SSFSE can be performed very quickly within single breath-hold, making it less susceptible to bowel movements than other sequences (9). Moreover, omitting the use of anti-peristaltic agents before performing SSFSE could be considered feasible. However, the possibility of image degradation owing to image blurring may affect diagnostic capability.
Recently, a deep learning-based reconstruction (DLR) pipeline (AIR Recon DL, GE Healthcare, Waukesha, USA) was used to provide high-quality images with reduced image blurring. The DLR pipeline utilizes raw k-space data as input and produces high-quality images as output, simultaneously with the MRI acquisition (10). Previous reports have demonstrated that DLR can improve image quality and reduce artifacts in T2-weighted abdominal imaging (11,12). Our hypothesis stated that DLR could compensate for image degradation attributed to the absence of an anti-peristaltic agent and provide higher-quality images compared to SSFSE with conventional reconstruction. Thus, we compared the image quality of single breath-hold SSFSE using conventional reconstruction and single breath-hold SSFSE using DLR with and without an anti-peristaltic agent and evaluated their diagnostic performance for active inflammation in Crohn’s disease.
Methods
This study was approved by the institutional review board of Inje University Haeundae Paik Hospital (No. HPIRB 2023-04-016-002). The requirement for informed consent was waived owing to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patients
This retrospective study included 45 patients (31 men, 14 women; mean age, 31.5±9.5 years) who underwent MRE for known Crohn’s disease between October 2021 and September 2022.
Image acquisition
MRE was performed using a 3T MR imaging (MRI) scanner (SIGNA™ Architect, GE Healthcare, Waukesha, USA) with two 30-channel coils (AIR™ anterior array coil, GE Healthcare, Waukesha, USA).
Prior to image acquisition, the patients ingested 1,000 mL of polyethylene glycol solution (Coolprep, Taejoon Pharm, Seoul, South Korea) to ensure adequate bowel distension. A total of 7.5 mg cimetropium bromide (Alpit, Hana Pharmaceutical Co., Seoul, Korea) was administered in three divided doses during the examination; the first dose was administered just before the second scan of the T2-weighted coronal image, the second dose was administered just before DWI, and the third dose was administered just before obtaining the coronal T1-weighted images.
Coronal SSFSE T2-weighted images without fat saturation were acquired within single breath-hold before and after anti-peristaltic agent administration, followed by image reconstruction using conventional reconstruction (CR) and DLR methods. The following four sets of data were generated: CR SSFSE with an antiperistaltic agent (CR-A), DLR SSFSE with an antiperistaltic agent (DLR-A), CR SSFSE without an antiperistaltic agent (CR-NA), and DLR SSFSE without an antiperistaltic agent (DLR-NA). The detailed imaging parameters are listed in Table S1.
DLR
Commercially available DLR pipeline (AIR™ Recon DL, GE Healthcare, Waukesha, USA) based on deep convolutional neural network (CNN). The DLR pipeline takes raw k-space data as its input and generates high fidelity images as its output. The CNN contains 4.4 million trainable parameters in approximately 10,000 kernels (10). The network was trained using pairs of images representing near perfect and conventional MRI images. DLR reduces truncation artifacts, increases sharpness, and offers tunable noise reduction levels. A reduction factor of 75% (DL High) was selected for this study. Our decision to apply the 75% (DL High) was substantiated through a comprehensive evaluation of noise reduction options, including 25% (DL Low) and 50% (DL Medium) levels, wherein we came to consensus that the 75% (DL High) was the most optimal for improving the image quality (10,13).
Image analysis
Two board-certified radiologists (with 2 and 12 years of abdominal radiology experience, respectively) who were blinded to the clinical information, except for the diagnosis of Crohn’s disease, independently reviewed the images. Two readers reviewed CR-A, DLR-NA, and CR-NA with a side-by-side comparison, using DLR-A as a reference standard. The readers evaluated the overall image quality and artifacts using the following 5-point scale: 1 = DLR-A is much better, 2 = DLR-A is slightly better, 3 = identical quality as DLR-A, 4 = slightly better quality than DLR-A, and much better quality than DLR-A (14,15). Both overall image quality and overall artifacts were subjectively assessed. In the evaluation of overall image quality, readers considered the image’s suitability for lesion detection, taking into account factors such as noise, artifacts, sharpness, and contrast. When assessing overall artifacts, readers examined the presence and extent of various issues, including motion artifacts caused by bowel peristalsis, ghosting artifacts, and FID artifacts.
Since reconstruction methods provide no benefit for bowel distension, the bowel distension degree was only evaluated using DLR-A and DLR-NA images. The extent of bowel distension was evaluated using the following grading scale: 1 = all bowel loops are collapsed without luminal separation, and the walls cannot be recognized; 2 = adequate bowel distension is visible in less than 50%; 3 = adequate bowel distension is visible in 50–80% of the segments; 4 = more than 80% of bowel loops are adequately distended; and 5 = all bowel loops in the segment are adequately distended. Adequate bowel distension was defined as bowel diameter more than 1.5 cm (16). For consistent interpretation, jejunal loops were defined as small bowel loops located on the upper and left sides of the abdominal wall, with thicker plicae circulares and densely arranged folds. The ileal loops were characterized by thinner circular plicae with loose arrangement, mainly in the lower compartment of the abdomen (16).
The parameters of active inflammation were assessed based on the bowel wall thickness, and mural and perimural signal intensity in the jejunum and ileum, respectively using a 4-point scale (17). This evaluation was conducted using only T2-weighed images. The detailed scoring system is presented in Table 1.
Table 1
| Parameter | Scoring | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Bowel wall thickness (mm) | 1–3 | >3–5 | >5–7 | >7 |
| Mural signal intensity on T2 weighted image | Equivalent to that of normal bowel wall | Minor increase in signal intensity | Moderate increase in signal intensity | Marked increase in signal intensity: bowel wall contains areas of high signal intensity, approaching that of the luminal content |
| Perimural signal intensity on T2 weighted images | Equivalent to that of normal mesentery | Increase in mesenteric signal intensity but no fluid collection | Small fluid rim (≤2 mm) | Large fluid rim (>2 mm) |
Each reader evaluated the sensitivity, specificity, and accuracy of the diagnosis of ileal and jejunal inflammation. Only the terminal ileum was selected to avoid overestimation of the diagnostic performance owing to multifocal involvement of the ileum. The reference standard for terminal ileal inflammation was determined by the endoscopic results obtained within one week prior to or following the MRE examination. For the jejunum, an abdominal radiologist reviewed all the available sequences to determine the presence of active inflammation in the jejunal loops, which were used as the reference standard.
The signal-to-noise ratio (SNR) was calculated in two slices, at the level of superior mesenteric artery (SMA) and iliac bifurcation of aorta. The mean signal of the image slice was calculated, and it was divided by estimated standard deviation of noise level. The noise level was estimated by a hybrid discrete wavelet transform (DWT) and edge information removal-based algorithm (18,19).
Statistical analysis
The scores for the overall image quality, artifacts, and bowel distension were compared using a paired-sample Wilcoxon signed-rank test. The inter-reader and inter-sequence agreement of bowel wall thickness, mural signal intensity, and perimural signal intensity were calculated using Cohen’s kappa coefficients (ĸ). The kappa value was interpreted as poor, fair, moderate, good, and excellent agreement for ĸ <0.2, 0.21–0.4, 0.41–0.60, 0.61–0.80, and 0.81–1.0, respectively. The SNR between four images were compared using the Friedman test. Statistical significance was set at P<0.05. The Bonferroni correction was used for multiple comparison in the SNR comparison. Statistical analyses were performed using SPSS Statistics for Windows, version 28.0 (IBM Corp., Armonk, NY, USA).
Results
Comparison of the qualitative scores
In terms of overall quality, DLR-NA demonstrated no significant difference in the scores compared with DLR-A (Reader 1: P=0.157; Reader 2: P=0.317). CR-NA and CR-A demonstrated significant differences compared to DLR-A (both readers, P<0.05). DLR-NA and DLR-A were mostly equivalent in terms of image quality for both readers (43 and 44 cases for Readers 1 and 2, respectively). However, when compared to CR-NA and CR-A, DLR-A demonstrated slightly better image quality in more cases (12 cases for CR-NA and eight cases for CR-A by Reader 1; 39 cases for CR-NA and 39 cases for CR-A by Reader 2).
Considering the overall artifacts, no significant difference was observed between DLR-NA and DLR-A for Reader 2 (P=0.317). Reader 1 found that DLR-A was slightly better than DLR-NA in four cases and identical in 41 cases (P=0.046). CR-NA and CR-A demonstrated significant differences in the overall artifacts when compared with DLR-A, with majority of the DLR-A images graded showing slightly better quality than the CR-NA or CR-A images (P<0.001). The detailed results are summarized in Table 2.
Table 2
| Measures | Images | Scoring scales | Number of patients | |
|---|---|---|---|---|
| Reader 1 | Reader 2 | |||
| Overall image quality | DLR-NA | DLR-A slightly better than DLR-NA | 2 | 1 |
| DLR-NA and DLR-A identical | 43 | 44 | ||
| DLR-NA slightly better than DLR-A | 0 | 0 | ||
| P value | 0.157 | 0.317 | ||
| CR-NA | DLR-A slightly better than CR-NA | 12 | 39 | |
| CR-NA and DLR-A identical | 33 | 6 | ||
| CR-NA slightly better than DLR-A | 0 | 0 | ||
| P value | 0.001 | <0.001 | ||
| CR-A | DLR-A slightly better than CR-A | 8 | 39 | |
| CR-A and DLR-A identical | 37 | 6 | ||
| CR-A slightly better than DLR-A | 0 | 0 | ||
| P value | 0.005 | <0.001 | ||
| Overall artifact | DLR-NA | DLR-A slightly better than DLR-NA | 4 | 1 |
| DLR-NA and DLR-A identical | 41 | 44 | ||
| DLR-NA slightly better than DLR-A | 0 | 0 | ||
| P value | 0.046 | 0.317 | ||
| CR-NA | DLR-A slightly better than CR-NA | 35 | 39 | |
| CR-NA and DLR-A identical | 10 | 6 | ||
| CR-NA slightly better than DLR-A | 0 | 0 | ||
| P value | <0.001 | <0.001 | ||
| CR-A | DLR-A slightly better than CR-A | 34 | 39 | |
| CR-A and DLR-A identical | 11 | 6 | ||
| CR-A slightly better than DLR-A | 0 | 0 | ||
| P value | <0.001 | <0.001 | ||
No scores were given for any qualitative measure of image quality, indicating that the DLR-A image was significantly better than the other images (DLR-NA, CR-NA, and CR-A) or that any of these other images were significantly better than DLR-A. DLR-NA, SSFSE with deep learning-based reconstruction without anti-peristaltic agent; CR-NA, SSFSE with conventional reconstruction without anti-peristaltic agent; CR-A, SSFSE with conventional reconstruction with anti-peristaltic agent; DLR-A, SSFSE with deep learning-based reconstruction with an anti-peristaltic agent; SSFSE, single-shot fast spin-echo.
Both readers found a statistically significant difference in the bowel distension score between DLR-A and DLR-NA in the jejunum [Reader 1: DLR-NA, 2.78±0.70 (mean ± standard deviation); DLR-A, 2.87±0.79; P=0.046; and Reader 2: DLR-NA, 2.73±0.72; DLR-A, 2.89±0.71; P=0.008], whereas no significant difference was observed in the ileal loop by either reader (Reader 1: DLR-NA, 3.44±0.59; DLR-A, 3.51±0.59; P=0.083; and Reader 2: DLR-NA, 3.98±0.66; DLR-A, 4.00±0.67; P=0.317). The results are presented in Table 3.
Table 3
| Bowel segment | Reader 1 | Reader 2 | |||||
|---|---|---|---|---|---|---|---|
| DLR-NA | DLR-A | P value | DLR-NA | DLR-A | P value | ||
| Jejunum | 2.78±0.70 | 2.87±0.79 | 0.046 | 2.73±0.72 | 2.89±0.71 | 0.008 | |
| Ileum | 3.44±0.59 | 3.51±0.59 | 0.083 | 3.98±0.66 | 4.00±0.67 | 0.317 | |
Values are presented as mean ± standard deviation. DLR-NA, SSFSE with deep learning-based reconstruction without anti-peristaltic agent; DLR-A, SSFSE with deep learning-based reconstruction with an anti-peristaltic agent; SSFSE, single-shot fast spin-echo.
Comparison of the inflammatory parameters
The inter-image agreement of the parameters of active inflammation in both readers demonstrated moderate to excellent agreement (Reader 1: ĸ =0.49–1.00; Reader 2: ĸ =1.00). The inter-reader agreement demonstrated moderate to good agreement (ĸ = 0.53–0.66 for all comparisons). The detailed results are presented in Tables 4,5.
Table 4
| Parameter | Image | |||
|---|---|---|---|---|
| DLR-NA | CR-NA | CR-A | DLR-A | |
| Jejunum | ||||
| Mural thickness | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) |
| Mural signal intensity | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) |
| Perimural signal intensity | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) | 0.656 (0.031–1.000) |
| Ileum | ||||
| Mural thickness | 0.621 (0.431–0.795) | 0.621 (0.431–0.795) | 0.588 (0.429–0.822) | 0.588 (0.429–0.822) |
| Mural signal intensity | 0.655 (0.485–0.831) | 0.655 (0.485–0.831) | 0.655 (0.521–0.855) | 0.655 (0.521–0.855) |
| Perimural signal intensity | 0.529 (0.286–0.697) | 0.529 (0.286–0.697) | 0.529 (0.286–0.697) | 0.529 (0.286–0.697) |
Values in parentheses are the 95% confidence intervals. DLR-NA, SSFSE with deep learning-based reconstruction without antiperistaltic agent; CR-NA, SSFSE with conventional reconstruction without antiperistaltic agent; CR-A, SSFSE with conventional reconstruction with antiperistaltic agent; DLR-A, SSFSE with deep learning-based reconstruction with an antiperistaltic agent; SSFSE, single-shot fast spin-echo.
Table 5
| Parameters | DLR-NA vs. CR-NA | DLR-NA vs. CR-A | DLR-NA vs. DLR-A | CR-NA vs. CR-A | CR-NA vs. DLR-A | CR-A vs. DLR-A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | ||||||
| Jejunum | |||||||||||||||||
| Mural thickness | 1.000 (1.000-1.000) |
1.000 (1.000–1.000) |
0.494 (0.483–0.505) |
1.000 (1.000–1.000) |
0.494 (0.483–0.505) |
1.000 (1.000–1.000) |
0.494 (0.483–0.505) |
1.000 (1.000–1.000) |
0.494 (0.483–0.505) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
|||||
| Mural signal intensity | 1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
|||||
| Perimural signal intensity | 1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
|||||
| Ileum | |||||||||||||||||
| Mural thickness | 1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
|||||
| Mural signal intensity | 1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
0.967 (0.906–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
|||||
| Perimural signal intensity | 1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
1.000 (1.000–1.000) |
|||||
Values in parentheses are the 95% confidence intervals. DLR-NA, SSFSE with deep learning-based reconstruction without antiperistaltic agent; CR-NA, SSFSE with conventional reconstruction without antiperistaltic agent; CR-A, SSFSE with conventional reconstruction with antiperistaltic agent; DLR-A, SSFSE with deep learning-based reconstruction with an antiperistaltic agent; SSFSE, single-shot fast spin-echo.
Diagnostic performance
The diagnostic performance for detecting active inflammation in the terminal ileum was evaluated in each image using endoscopic findings within one week as the reference standard. Endoscopic results were available for 29 patients (7 males, 22 females; mean age ± standard deviation, 30.9±9.3 years, ranged 16–52 years), with 18 of them having active inflammation in the terminal ileum. The average interval between the endoscopy and MRE was 2.9±2.53 days (range, 0–7 days). The sensitivity, specificity, and accuracy of DLR-A were 94.42%, 81.83%, and 89.69%; and 83.33%, 90.91%, and 86.21% for Readers 1 and 2, respectively. The presence of active inflammation assessment did not vary among the four image sets; therefore, the diagnostic performance was the same for DLR-NA, CR-A, and CR-NA, as it was for DLR-A (Figure 1).
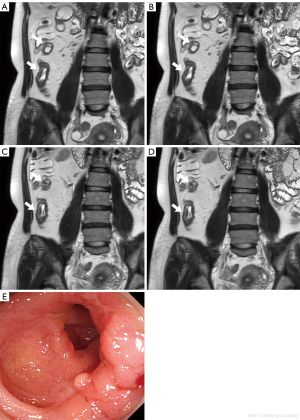
Among 45 patients, 2 showed active inflammation in the jejunum according to the reference standard. The sensitivity, specificity, and accuracy of DLR-A in the jejunal loop were 50%, 100%, and 97.8%; and 100%, 100%, and 100% for Readers 1 and 2, respectively. It should be noted that active inflammation was only observed in two cases of the jejunal loop, which may have contributed to the low sensitivity findings for Reader 1 (Figures 2,3). The diagnostic performance for active inflammation in jejunum was also the same among four image sets.


SNR
At both the SMA and iliac bifurcation levels, DLR-A (mean values of 117.15±24.19 in the SMA level and 120.80±18.42 in the bifurcation) and DLR-NA (120.28±24.63 in the SMA and 123.05±23.98 in the bifurcation) exhibited significantly higher SNR when compared to CR-NA (63.99±12.24 in the SMA and 61.46±12.35 in the bifurcation) and CR-A (82.99±21.63 in the SMA and 80.38±19.77 in the bifurcation). There were no significant differences between DLR-A and DLR-NA, but significant differences between CR-A and CR-NA in both the SMA and iliac bifurcation levels. The summarized results are in Table 6.
Table 6
| SNR measured level | DLR-NA | DLR-A | CR-NA | CR-A |
|---|---|---|---|---|
| SMA level (mean ± SD) | 120.28±24.63 | 117.15±24.19* | 63.99±12.24 | 82.99±21.63 |
| Bifurcation level (mean ± SD) | 123.05±23.98 | 120.80±18.42* | 61.46±12.35 | 80.38±19.77 |
*, all comparison except for DLR-NA and DLR-A showed significant difference in signal-to-noise ratio in both SMA and bifurcation level (P<0.001). CR-A, SSFSE with conventional reconstruction with anti-peristaltic agent; CR-NA, SSFSE with conventional reconstruction without anti-peristaltic agent; DLR-NA, SSFSE with deep learning-based reconstruction without anti-peristaltic agent; DLR-A, SSFSE with deep learning-based reconstruction with an anti-peristaltic agent; SNR, Signal-to-noise ratio; SD, standard deviation; SMA, superior mesenteric artery; SSFSE, single-shot fast spin-echo.
Discussion
This study compared the image quality and diagnostic performance of four single breath-hold SSFSE image sets for patients with known Crohn’s disease: namely conventional reconstruction with and without an anti-peristaltic agent, and deep learning-based reconstruction with and without an anti-peristaltic agent. When compared with DLR-A, DLR-NA demonstrated no significant difference in the overall image quality and artifacts, whereas DLR-A showed better image quality and fewer artifacts than both CR-A and CR-NA. Furthermore, DLR images showed significantly high SNR compared with CR images. The diagnostic performance for detecting active inflammation did not vary among the four image sets. Inter-reader and inter-image agreement of the parameters of active inflammation was fair to excellent. These results suggest that the effect of an anti-peristaltic agent on the image quality and artifacts of SSFSE in MRE is not as profound as that of DLR. Furthermore, the interpretation of parameters and diagnostic accuracy for active inflammation in Crohn’s disease did not change with different reconstruction methods or with the omission of an anti-peristaltic agent.
Most studies on MRE have been conducted using MRE that incorporates anti-peristaltic agents. Although a few studies have reported the feasibility of performing MRE without an anti-peristaltic agent (2,20), the current guidelines recommend its use (3,4). Nevertheless, limited evidence exists on the effect of anti-peristaltic agents on the image quality of MRE or active inflammation diagnosis. In a previous study comparing MRE without anti-peristaltic agents to CT enterography (CTE), significant image degradation was observed in MRE without an anti-peristaltic agent; however, the diagnostic interpretation for the presence of active inflammation demonstrated substantial agreement with CTE (2). Another study examining the use of anti-peristaltic agents for DWI suggested that anti-peristaltic agents are necessary for DWI acquisition because omitting them could reduce the sensitivity of the diagnosis of active inflammation (8). This is the first study to investigate the effect of anti-peristaltic agents on the image quality and diagnostic performance of T2WI in MRE. Our results indicate that the omission of the use of anti-peristaltic agents did not affect the diagnosis of active inflammation upon T2WI of MRE.
DLR demonstrated improved image quality and reduced artifacts in SSFSE T2WI of abdominal MRI in previous studies (10,11). Our study further confirms the benefits of DLR in improving image quality and reducing artifacts compared to CR. Furthermore, DLR revealed a more significant impact on image quality than the use of an anti-peristaltic agent for SSFSE of MRE. This is because SSFSE is relatively robust to motion, because each image is obtained within a single echo train; however, it possesses certain inherent limitations, such as reduced SNR owing to short acquisition time, blurring, and reduced image contrast due to T2 decay during the extended echo train (21). DLR can reduce these factors that cause image degradation. Additionally, peristaltic motility is decreased in the inflamed bowels of patients with Crohn’s disease (22,23).
The protocols for the use of anti-peristaltic agents vary among institutions. Various anti-peristaltic agents are used, with glucagon being commonly used in the United States, and hyoscine butylbromide also is widely used. Additionally, hyoscine, hyoscyamine sulfate, cimetropium bromide, among others, can be used for anti-peristaltic effect (1). The administration route varies and includes intramuscular, intravenous routes, as well as sublingual administration. The majority of these medications have a rapid onset of action, typically within 1 to 2 min (24,25). Notably, the two most commonly utilized agents, glucagon and hyoscine butylbromide, have relatively short durations of effect (1,24). A previous study demonstrated that split-dose administration results in significantly decreased peristalsis compared with single-dose administration (5). Current guidelines recommend the administration of an anti-peristaltic agent as a split dose (4). At our institution, we administer anti-peristaltic agents as split doses at three different times: before T2WI, DWI, and contrast enhancement. Upon considering the results of our study, the administration of an anti-peristaltic agent before T2WI can be omitted, while preserving the image quality and diagnostic performance with the help of DLR. Although the omitted dose is small, it is important for making the examination more convenient with lower probability of side effects.
Regarding bowel distension, both the readers reported that the jejunum demonstrated significantly less distension in the images generated without the use of anti-peristaltic agents, and both readers found no difference in the ileal distension. Although significant difference was noted in both readers, the difference in the distension grade of the jejunum between image sets with and without anti-peristaltic agents was not large (without anti-peristalsis group: Reader 1, 2.78±0.70; Reader 2, 2.73±0.72; and with anti-peristalsis group: Reader 1, 2.87±0.79; Reader 2 2.89±0.71), suggesting that the jejunal loop distension is still insufficient even following the use of an anti-peristaltic agent. Fortunately, no significant clinical impact is observed concerning the diagnosis of active inflammation in Crohn’s disease because the prevalence of CD in the jejunum is low (26,27). However, anti-peristaltic agents should be used in patients with suspected or previous history of inflammation in the jejunum.
Our study has certain limitations. First, it was a retrospective study. Second, some patients did not undergo ileocolonoscopy; therefore, they were excluded from the analysis of the diagnostic performance in the terminal ileum. Third, the readers could not be completely blinded to the image sets owing to the possibility of discerning the image sets according to different textures and distension of the bowel loops. Lastly, we scored image quality subjectively, but did not perform quantitative analyses such as measuring the SNR in this study. However, our very recent study demonstrated that DLR significantly increased the SNR of SSFSE T2WI in MR enterography (9).
Conclusions
DLR improved the image quality and reduced artifacts in single breath-hold SSFSE T2-weighted MRE images more significantly than the use of an anti-peristaltic agent. Eliminating the use of an anti-peristaltic agent before SSFSE T2WI acquisition and adding DLR can be performed without affecting the image quality and diagnostic performance for active inflammation in patients with Crohn’s disease. This result in a more convenient examination in terms of time and cost, with reducing the chance of exposure to potential side effects of anti-peristaltic agents.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-738/coif). J.L. is a current employee of GE HealthCare Korea, and his contribution was limited to assisting with statistical analysis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Inje University Haeundae Paik Hospital (No. HPIRB 2023-04-016-002) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chatterji M, Fidler JL, Taylor SA, Anupindi SA, Yeh BM, Guglielmo FF. State of the Art MR Enterography Technique. Top Magn Reson Imaging 2021;30:3-11. [Crossref] [PubMed]
- Grand DJ, Beland MD, Machan JT, Mayo-Smith WW. Detection of Crohn's disease: Comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. Eur J Radiol 2012;81:1735-41. [Crossref] [PubMed]
- Grand DJ, Guglielmo FF, Al-Hawary MM. MR enterography in Crohn's disease: current consensus on optimal imaging technique and future advances from the SAR Crohn's disease-focused panel. Abdom Imaging 2015;40:953-64. [Crossref] [PubMed]
- Taylor SA, Avni F, Cronin CG, Hoeffel C, Kim SH, Laghi A, Napolitano M, Petit P, Rimola J, Tolan DJ, Torkzad MR, Zappa M, Bhatnagar G, Puylaert CAJ, Stoker J. The first joint ESGAR/ ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur Radiol 2017;27:2570-82. [Crossref] [PubMed]
- Rao A, Sitheeque F, Gustafson S, Lu M, Prior M. MR enterography - Impact on image quality between single- versus split-dose Buscopan. J Med Imaging Radiat Oncol 2020;64:331-7. [Crossref] [PubMed]
- Seo N, Park SH, Kim KJ, Kang BK, Lee Y, Yang SK, Ye BD, Park SH, Kim SY, Baek S, Han K, Ha HK. MR Enterography for the Evaluation of Small-Bowel Inflammation in Crohn Disease by Using Diffusion-weighted Imaging without Intravenous Contrast Material: A Prospective Noninferiority Study. Radiology 2016;278:762-72. [Crossref] [PubMed]
- Kim J, Seo N, Bae H, Kang EA, Kim E, Chung YE, Lim JS, Kim MJ. Comparison of Sensitivity Encoding (SENSE) and Compressed Sensing-SENSE for Contrast-Enhanced T1-Weighted Imaging in Patients With Crohn Disease Undergoing MR Enterography. AJR Am J Roentgenol 2022;218:678-86. [Crossref] [PubMed]
- Park SH, Huh J, Park SH, Lee SS, Kim AY, Yang SK. Diffusion-weighted MR enterography for evaluating Crohn's disease: Effect of anti-peristaltic agent on the diagnosis of bowel inflammation. Eur Radiol 2017;27:2554-62. [Crossref] [PubMed]
- Park EJ, Lee Y, Lee J. Impact of Deep-Learning Based Reconstruction on Single-Breath-Hold, Single-Shot Fast Spin-Echo in MR Enterography for Crohn’s Disease. J Korean Soc Radiol 2023;84:e93. [Crossref] [PubMed]
- Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:200806559 2020.
- Sheng RF, Zheng LY, Jin KP, Sun W, Liao S, Zeng MS, Dai YM. Single-breath-hold T2WI liver MRI with deep learning-based reconstruction: A clinical feasibility study in comparison to conventional multi-breath-hold T2WI liver MRI. Magn Reson Imaging 2021;81:75-81. [Crossref] [PubMed]
- Wang X, Ma J, Bhosale P, Ibarra Rovira JJ, Qayyum A, Sun J, Bayram E, Szklaruk J. Novel deep learning-based noise reduction technique for prostate magnetic resonance imaging. Abdom Radiol (NY) 2021;46:3378-86. [Crossref] [PubMed]
- Koch KM, Sherafati M, Arpinar VE, Bhave S, Ausman R, Nencka AS, Lebel RM, McKinnon G, Kaushik SS, Vierck D, Stetz MR, Fernando S, Mannem R. Analysis and Evaluation of a Deep Learning Reconstruction Approach with Denoising for Orthopedic MRI. Radiol Artif Intell 2021;3:e200278. [Crossref] [PubMed]
- Liu P, Wang Q, Peng C, Luo B, Zhang J. Combined application of isotropic three-dimensional fast spin echo (3D-FSE-Cube) with 2-point Dixon fat/water separation (FLEX) and 3D-FSE-cube in MR dacryocystography. Br J Radiol 2019;92:20180157. [Crossref] [PubMed]
- Hahn S, Yi J, Lee HJ, Lee Y, Lee J, Wang X, Fung M. Comparison of deep learning-based reconstruction of PROPELLER Shoulder MRI with conventional reconstruction. Skeletal Radiol 2023;52:1545-55. [Crossref] [PubMed]
- Lee SB, Kim SH, Son JH, Baik JY. Evaluation of bowel distension and bowel wall visualization according to patient positions during administration of oral contrast media for CT enterography. Br J Radiol 2017;90:20170352. [Crossref] [PubMed]
- Steward MJ, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S, Novelli M, Halligan S, Rodriguez-Justo M, Taylor SA. Non-perforating small bowel Crohn's disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol 2012;81:2080-8. [Crossref] [PubMed]
- Pimpalkhute VA, Page R, Kothari A, Bhurchandi KM, Kamble VM. Digital Image Noise Estimation Using DWT Coefficients. IEEE Trans Image Process 2021;30:1962-72. [Crossref] [PubMed]
- Son JH, Lee Y, Lee HJ, Lee J, Kim H, Lebel MR. LAVA HyperSense and deep-learning reconstruction for near-isotropic (3D) enhanced magnetic resonance enterography in patients with Crohn's disease: utility in noise reduction and image quality improvement. Diagn Interv Radiol 2023;29:437-49. [Crossref] [PubMed]
- Grand DJ, Kampalath V, Harris A, Patel A, Resnick MB, Machan J, Beland M, Chen WT, Shah SA. MR enterography correlates highly with colonoscopy and histology for both distal ileal and colonic Crohn's disease in 310 patients. Eur J Radiol 2012;81:e763-9. [Crossref] [PubMed]
- Bhosale P, Ma J, Choi H. Utility of the FIESTA pulse sequence in body oncologic imaging: review. AJR Am J Roentgenol 2009;192:S83-93 (Quiz S94-7).
- Bickelhaupt S, Pazahr S, Chuck N, Blume I, Froehlich JM, Cattin R, Raible S, Bouquet H, Bill U, Rogler G, Frei P, Boss A, Patak MA. Crohn's disease: small bowel motility impairment correlates with inflammatory-related markers C-reactive protein and calprotectin. Neurogastroenterol Motil 2013;25:467-73. [Crossref] [PubMed]
- Bickelhaupt S, Froehlich JM, Cattin R, Patuto N, Tutuian R, Wentz KU, Culmann JL, Raible S, Bouquet H, Bill U, Patak MA. Differentiation between active and chronic Crohn's disease using MRI small-bowel motility examinations - initial experience. Clin Radiol 2013;68:1247-53. [Crossref] [PubMed]
- Froehlich JM, Daenzer M, von Weymarn C, Erturk SM, Zollikofer CL, Patak MA. Aperistaltic effect of hyoscine N-butylbromide versus glucagon on the small bowel assessed by magnetic resonance imaging. Eur Radiol 2009;19:1387-93. [Crossref] [PubMed]
- Dillman JR, Smith EA, Khalatbari S, Strouse PJ. I.v. glucagon use in pediatric MR enterography: effect on image quality, length of examination, and patient tolerance. AJR Am J Roentgenol 2013;201:185-9. [Crossref] [PubMed]
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet 2017;389:1741-55. [Crossref] [PubMed]
- Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology 2013;145:158-165.e2. [Crossref] [PubMed]


