
Predicting epithelial ovarian cancer prognosis: correlation of posttreatment 18F-fluorodeoxyglucose positron emission tomography-computed tomography metabolic parameters, serum carbohydrate antigen, and human epididymis protein levels with overall survival
Introduction
Among gynecological malignancies, ovarian cancer (OC) has the third highest incidence and is the leading cause of death (1,2), with approximately 314,000 new cases and 207,123 mortalities worldwide in 2020 and a 5-year relative survival rate estimated at 48.6% (3). Epithelial OC (EOC) is the most prevalent type of ovarian malignancy, and its histologic subtypes include plasmacytoma, mucinous carcinoma, endometrioid carcinoma, and clear cell carcinoma (4). Although most patients with EOC reach a state of maximum clinical remission through surgery (either staged or cytoreductive) combined with platinum-based chemotherapy, the prognosis remains poor (5). This is because approximately 70% of patients with OC experience recurrence or metastasis within 5 years, highlighting the persistent challenge faced by these patients. The prognosis of patients with OC is a crucial factor in guiding their treatment strategy. Consequently, optimal surveillance in the monitoring of disease progression in patients with OC is essential. However, there is no consensus regarding the optimal method and timing for follow-up. The chief goal in the early detection of recurrent metastases and proactive intervention in clinical practice is to improve patient survival, and this requires an optimized approach in monitoring disease response and in obtaining prognostic information for guiding the treatment of patients with OC.
The role of various tumor markers in OC management is significant, with changes in their levels serving as indicators of disease progression and status. Carbohydrate antigen 125 (CA125) is a prevalent biomarker extensively utilized as an early diagnostic tool and for identifying recurrent metastases of OC (6-9). However, the specificity and sensitivity of CA125, when tested individually, have been reportedly to be fairly suboptimal (10). After CA125, another notable biomarker, human epididymis protein 4 (HE4), a serine protease inhibitor, received US Food and Drug Administration (FDA) approval as a diagnostic and disease recurrence monitoring tool (11-13). Studies indicate that HE4 may have superior specificity and sensitivity compared to CA125 (10). Nonetheless, traditional serum tumor marker assays, despite their in vitro diagnostic capability, are limited in accurately localizing tumor recurrence and metastasis, rendering them less informative for secondary surgery indications.
18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET/CT) has significantly benefited the management of patients with cancer, offering functional metabolic information, quantifiable data, and morphological insights (14,15). Metrics such as maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) are included in the PET/CT assessment. Notably, MTV and TLG, as volume-based parameters, represent the metabolic activity across the tumor. Furthermore, whole-body MTV (WBMTV) and whole-body TLG (WBTLG) account for the metabolic burden of all lesions, offering a comprehensive view of the tumor’s functional activity (16,17). Lee et al. reported TLG to be an independent predictor of progression-free survival (PFS) (18), but this observation was based on baseline PET/CT before treatment. It should be noted that pretreatment baseline PET/CT is not available for a substantial number of clinical cases due to various reasons.
The aim of this study was to determine the correlation between posttreatment quantitative 18F-FDG PET/CT parameters and serum markers and to clarify their potential prognostic implications for overall survival (OS) in patients diagnosed with EOC. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-859/rc).
Methods
Patients
This retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No. K 2023-140). The requirement for informed consent was waived due to the retrospective nature of the study. From May 2014 to May 2019, 215 patients with OC underwent their first posttreatment 18F-FDG PET/CT examination at the PET/CT Examination Center of the First Hospital of Chongqing Medical University. The selection criteria for patients in this study included the following: (I) histopathologically verified diagnosis of EOC; (II) completed initial treatment regimen of surgery and chemotherapy; (III) no history of dual primary cancers within 5 years preceding participation in the study; and (IV) comprehensive clinical and imaging data as well as serum CA125 and HE4 levels assessed within 2 weeks following PET/CT. Meanwhile, the exclusion criteria were as follows: (I) a primary treatment approach other than surgery with chemotherapy; (II) administration of radiotherapy or chemotherapy at the time of PET/CT; and (III) a lack of adequate follow-up data. After these criteria were applied, 79 patients were enrolled into the study (Figure 1). A thorough review of the medical records of all included patients was performed to extract relevant data, including age, International Federation of Gynecology and Obstetrics (FIGO) stage, histopathology, post-PET/CT treatment, serum CA125 and HE4 levels posttreatment, and whole-body metabolic parameters derived from PET/CT. Post-PET scan follow-ups were scheduled every 3–6 months and comprised medical history evaluation, physical assessment, serological examinations, and radiological imaging. The study’s primary endpoint was OS, defined as the time elapsed from the first posttreatment PET/CT examination until death or the follow-up termination in February 2023.
Tumor markers
All patients’ serum CA125 and HE4 levels were measured within 2 weeks after the PET/CT examination. Venous blood (3–5 m) was collected from patients on an empty stomach and sent to the laboratory for specimen pretreatment, with the procedure being carried out in strict accordance with the instrumentation manual. Our hospital uses the Architect i2000 fully automated microparticle chemiluminescence immunoassay analyzer (Abbot, Chicago, USA) and ancillary test kits, standards, and quality control products for testing. The reference values for serum CA125 and HE4 in our hospital’s laboratory department were chosen as grouping boundaries. Serum CA125 <35 U/mL was defined as negative and ≥35 U/mL as positive; serum HE4 <140 pmol/L was defined as negative and ≥140 pmol/L as positive.
18F-FDG PET/CT scanning and image analysis
18F-FDG PET/CT was conducted by the Medical Cyclotron of the Department of Nuclear Medicine, the First Hospital of Chongqing Medical University, with a Gemini TF 64 PET/CT detection system (Philips Medical Systems, Amsterdam, the Netherlands) and a radiochemical purity of >95%. 18F-FDG (3.70–5.55 MBq/kg) was administered intravenously to all patients who had fasted for at least 6 hours before the examination and who had a fasting blood glucose level below 6.1 mmol/L. Patients underwent PET/CT imaging from the apex of the head to the base of the thigh, during which time they were instructed be still and silent for an hour, with provisions to hydrate and urinate as needed. The PET scan parameters were as follows: 4D acquisition, layer thickness 4.0 mm, 5 min/bed for parts below the head and neck, 3 min/bed for the head and neck. Meanwhile, the CT scan specifications were as follows: 120 kV voltage, 100 mA current, and 4.0 mm layer thickness; scan data were corrected for attenuation; reconstruction images were iterated to obtain maximum intensity projection and fusion images; and the acquired sagittal, coronal, and axial images were transmitted to the Extended Brilliance Workstation (EBW) workstation (Philips).
All 18F-FDG PET/CT images were analyzed via a combination of visual interpretation and semiquantitative techniques by two senior nuclear medicine physicians with more than 10 years of experience in FDG PET/CT analysis who were blind to the clinical data but were familiar with the fundamentals of the patient and the rationale for the 18F-FDG PET/CT examination. Any disagreement between the two physicians was resolved via discussion. PET-positive (PET-P) status was defined as increased FDG uptake beyond that observed in the adjacent background that was consistent with a recurrent metastatic route linked to EOC. In higher physiologic FDG uptake areas, asymmetrically increased uptake was evaluated as positive. PET-negative (PET-N) status was characterized by no substantial increase in FDG uptake outside the physiological uptake region. The region of interest was automatically delineated on the PET/CT fusion image using 40% of the SUVmax as the threshold, and then the layers were manually modified layer by layer according to the contour of the lesion. The SUVmax, mean standardized uptake value (SUVmean), and MTV of the region of interest were automatically measured and then recorded, and the TLG was calculated (TLG = MTV × SUVmean). Additionally, WBSUVmax was selected as the maximum of all lesions in the patient, and WBMTV and WBTLG were the sum of all lesions. If patients with EOC had several posttreatment PET/CT examinations, the first scan was used for analysis, and the median time from completion of initial treatment to PET/CT scan was 11 months (range, 1–87 months).
Statistical analysis
Statistical analysis for this study was conducted using SPSS 26.0 software (IBM Corp., New York, USA). Statistical significance was defined as a P value of less than 0.05 based on a two-sided statistical test. Clinicopathological characteristics, serological markers, and 18F-FDG PET/CT parameters were expressed using descriptive statistics. Continuous variables were reported as medians and ranges, while categorical data were reported as percentages. Normality tests were conducted on the continuous variables, with the natural logarithm transformation applied to CA125, HE4, WBSUVmax, WBMTV, and WBTLG values. Differences in tumor markers between the PET-P and PET-N groups were assessed using the Mann-Whitney test. Pearson correlation coefficient was employed to evaluate linearity between serum CA125 and HE4 levels and PET/CT parameters. Survival analysis was performed using Kaplan-Meier, log-rank, and Cox proportional hazards regression analyses. These analyses were conducted separately for all patient groups and PET-P patient groups, with variables with a P value of <0.1 being included in a multivariate Cox regression analysis model. The model’s performance was evaluated using the Harrell-C concordance statistic. Survival curves were plotted for the entire patient cohort based on the presence or absence of 18F-FDG uptake, with additional curves drawn for variables included in the multivariate analysis. PET parameters were used to delineate two groups based on the median values.
Results
Patient characteristics
The patient characteristics are shown in Table 1. All patients underwent surgery (staged or tumor cytoreductive) combined with platinum-based chemotherapy and achieved maximum clinical remission. A total of 79 patients with EOC with a median age of 51 (range, 29–73) years were enrolled. The distribution of FIGO stage among patients was as follows: 3 cases were FIGO stage I, 24 were FIGO stage II, 42 were FIGO stage III, and 10 were FIGO stage IV. As for the histological type, 64 cases were plasmacytoma, 3 were mucinous carcinoma, 7 were endometrioid carcinoma, and 5 were clear cell carcinoma. Moreover, 63 patients showed FDG uptake on 18F-FDG PET/CT images, and 16 patients showed no FDG uptake. The post-PET/CT tumor-specific treatment across all participants was as follows: 22 patients (27.85%) underwent secondary surgery combined with chemotherapy, 41 patients (51.90%) received a single treatment, and 16 patients (20.25%) declined any tumor-specific treatment. When the PET-P and PET-N groups were compared, a larger proportion of patients in the PET-P group were at advanced disease stages, whereas most of the PET-N group were at the early stages. The OS rate for the PET-P group was considerably lower than that of the PET-N group, in which no deaths were recorded. Across the entire patient cohort, the median follow-up duration was 43 months, with a range of 2 to 99 months.
Table 1
| Characteristic | All patients (n=79) | PET findings | |
|---|---|---|---|
| Positive (n=63) | Negative (n=16) | ||
| Age (year) | 51.00 [29–73] | 53.00 [39–73] | 48.50 [29–62] |
| Status | |||
| Alive | 34 (43.04) | 18 (28.57) | 16 (100.00) |
| Dead | 45 (56.96) | 45 (71.43) | 0 |
| FIGO stage | |||
| Stage I–II | 27 (34.18) | 15 (23.81) | 12 (75.00) |
| Stage III–IV | 52 (65.82) | 48 (76.19) | 4 (25.00) |
| Histopathology | |||
| Serous | 64 (81.01) | 50 (79.37) | 14 (87.50) |
| Not serous | 15 (18.99) | 13 (20.63) | 2 (12.50) |
| Therapy after PET | |||
| Reoperation + chemo | 22 (27.85) | 19 (30.16) | 3 (18.75) |
| Another | 57 (72.15) | 44 (69.84) | 13 (81.25) |
| CA125 (U/mL) | 58.60 [4.20–1,746.60] | 83.80 [6.30–1,746.60] | 11.65 [4.20–363.20] |
| Negative (<35) | 28 (35.44) | 15 (23.81) | 13 (81.25) |
| Positive (≥35) | 51 (64.56) | 48 (76.19) | 3 (18.75) |
| HE4 (pmol/L) | 73.00 [18.00–644.00] | 91.00 [24.00–644.00] | 44.50 [18.00–130.00] |
| Negative (<140) | 50 (63.29) | 43 (68.25) | 7 (43.75) |
| Positive (≥140) | 29 (36.71) | 20 (31.75) | 9 (56.25) |
| PET parameters | |||
| WBSUVmax (g/mL) | 6.49 [0.00–27.91] | 8.24 [1.57–27.91] | – |
| WBMTV (mL) | 4.16 [0.00–194.75] | 6.72 [0.13–194.75] | – |
| WBTLG (g) | 14.71 [0.00–1,889.09] | 27.75 [0.20–1,889.09] | – |
| Median OS (month) | 43.00 [2–99] | 33.00 [2–99] | 76.00 [49–94] |
Data are presented as n (%) or median [range] of corresponding variables. PET, positron emission tomography; FIGO, The International Federation of Gynecology and Obstetrics; chemo, chemotherapy; CA125, carbohydrate antigen 125; HE4, human epididymis protein 4; WBSUVmax, whole-body maximum standardized uptake value; WBMTV, whole-body metabolic tumor volume; WBTLG, whole-body total lesion glycolysis; OS, overall survival.
18F-FDG PET/CT results and OS
Based on posttreatment PET imaging, the log-rank test revealed a significant difference in OS between the PET-P and PET-N patient groups (P<0.001; Figure 2). According to univariate Cox regression analysis, PET-P patients had a significantly shorter OS, [hazard ratio (HR) =40.177, 95% confidence interval (CI): 2.690–600.134; P=0.007] (Table 2). The mean OS of the PET-P group was 33 months, while that of the PET-N group was 76 months (Table 1).

Table 2
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| PET findings | |||
| Positive | 40.177 | 2.690, 600.134 | 0.007* |
| Negative† | 1.000 | – | – |
| Histopathology | |||
| Serous | 0.696 | 0.343, 1.411 | 0.314 |
| Not serous† | 1.000 | – | – |
| FIGO stage | |||
| Stage I–II | 0.321 | 0.153, 0.675 | 0.003* |
| Stage III–IV† | 1.000 | – | – |
| CA125 (U/mL) | |||
| Positive (≥35) | 2.932 | 1.405, 6.116 | 0.004* |
| Negative (<35)† | 1.000 | – | – |
| HE4 (pmol/L) | |||
| Positive (≥140) | 2.801 | 1.520, 5.163 | 0.001* |
| Negative (<140)† | 1.000 | – | – |
| Age (year) | 1.028 | 0.994, 1.064 | 0.112 |
*, a significance of P<0.05. †, reference group. OS, overall survival; HR, hazard ratio; CI, confidence interval; PET, positron emission tomography; FIGO, The International Federation of Gynecology and Obstetrics; CA125, carbohydrate antigen 125; HE4, human epididymis protein 4.
Univariate Cox regression analysis comprising clinical variables revealed that other characteristics significantly related to OS included FIGO stage (HR =0.321, 95% CI: 0.153–0.675; P=0.003), CA125 level (HR =2.932, 95% CI: 1.405–6.116; P=0.004), and HE4 level (HR =2.801, 95% CI: 1.520–5.163; P=0.001). The age and histological type of the patients had no significant correlation with OS (Table 2). The 1- and 3-year survival rates were 92.6% and 77.8% for patients with early-stage FIGO grade and 80.8% and 44.2% for those with advanced-stage FIGO grade , respectively; the 1- and 3-year survival rates for CA125-negative (CA125-N) patients were 89.3% and 71.4%, respectively, while those for CA125-positive (CA125-P) patients, were 82.4% and 47.1%, respectively; the 1- and 3-year survival rates for HE4-negative (HE4-N) patients were 93.2% and 68.2%, respectively, while those for HE4-positive (HE4-P) patients were 74.3% and 40.0%, respectively.
FDG-positive uptake group and recurrence site
In the PET-P group, 205 recurrent metastatic lesions were identified in 63 patients. The most common site of disease was lymph node metastasis (n=83, 40.49%), followed by peritoneal carcinomatosis (n=61, 29.76%) and pelvic tumors (n=34, 16.59%). There were five vaginal cuff recurrences. Parenchymal solid organ metastases were observed in the liver (n=10, 4.88%), spleen (n=3, 1.46%), lung (n=2, 0.10%), pancreas (n=1, 0.05%), and adrenal gland (n=1, 0.05%). In addition to this, two patients (0.98%) had pleural lesions, and one patient (0.49%) had abdominal wall lesions; moreover, two patients had lesions on the vertebrae.
FDG-positive uptake group and OS
In the univariate analysis, OS was found to be substantially correlated with lnWBMTV (HR =1.424, 95% CI: 1.115–1.819; P=0.005), lnWBTLG (HR =1.362, 95% CI: 1.099–1.688; P=0.005), and therapy after PET (HR =0.340, 95% CI: 0.162–0.715; P=0.004), (Table 3). LnWBMTV, lnWBTLG, therapy after PET, and HE4 levels were included in the multivariate Cox regression analysis for variables with P<0.1. Since TLG was calculated as the product of MTV and SUVmean, the multicollinearity between lnWBMTV and lnWBTLG was evaluated before multivariate analysis. Pearson correlation coefficient analysis indicated a highly positive relationship (r=0.970; P<0.001); therefore, lnWBMTV and lnWBTLG were separately analyzed with other variables. Model 1 refers to the analysis using lnWBMTV, and Model 2 refers to the analysis using lnWBTLG. Multivariate analysis showed that lnWBMTV (HR =1.309, 95% CI: 1.037–1.651; P=0.023) and lnWBTLG (HR =1.312, 95% CI: 1.070–1.609; P=0.009) were significantly associated with patient OS (Table 4). Therapy after PET was a significant OS-related variable in both Model 1 (HR =0.393, 95% CI: 0.180–0.857, P=0.019) and Model 2 (HR =0.367, 95% CI: 0.168–0.800, P=0.012) (Table 4). The C-statistic indexes for Models 1 and 2 were 0.723 and 0.727, respectively.
Table 3
| Variable | Univariable | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| PET measure | |||
| lnWBSUVmax | 1.470 | 0.913, 2.378 | 0.113 |
| lnWBMTV | 1.424 | 1.115, 1.819 | 0.005* |
| lnWBTLG | 1.362 | 1.099, 1.688 | 0.005* |
| Therapy after PET | |||
| Reoperation+ chemo | 0.340 | 0.162, 0.715 | 0.004* |
| Another† | 1.000 | ||
| Histopathology | |||
| Serous | 0.890 | 0.423, 1.871 | 0.758 |
| Not serous† | 1.000 | ||
| FIGO stage | |||
| Stage I–II | 0.581 | 0.276, 1.227 | 0.155 |
| Stage III–IV† | 1.000 | ||
| CA125 (U/mL) | |||
| Positive (≥35) | 1.250 | 0.600, 2.604 | 0.552 |
| Negative (<35)† | 1.000 | ||
| HE4 (pmol/L) | |||
| Positive (≥140) | 1.770 | 0.963, 3.251 | 0.066 |
| Negative (<140)† | 1.000 | ||
| Age (year) | 1.004 | 0.969, 1.041 | 0.811 |
*, a significance of P<0.05. †, reference group. OS, overall survival; PET, positron emission tomography; PET-P, PET positive; HR, hazard ratio; CI, confidence interval; ln, natural logarithm; WBSUVmax, whole-body maximum standardized uptake value; WBMTV, whole-body metabolic tumor volume; WBTLG, whole-body total lesion glycolysis; chemo, chemotherapy; FIGO, The International Federation of Gynecology and Obstetrics; CA125, carbohydrate antigen 125; HE4, human epididymis protein 4.
Table 4
| Variable | Multivariable (Model 1) | Multivariable (Model 2) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| lnWBMTV | 1.309 (1.037, 1.651) | 0.023* | – | – | |
| lnWBTLG | – | – | 1.312 (1.070, 1.609) | 0.009* | |
| Therapy after PET | |||||
| Reoperation + chemo | 0.393 (0.180, 0.857) | 0.019* | 0.367 (0.168, 0.800) | 0.012* | |
| Another† | 1.000 | – | |||
| HE4 (pmol/L) | 1.186 (0.620, 2.267) | 0.606 | 1.210 (0.634, 2.309) | 0.564 | |
| C-index | 0.723 | 0.727 | |||
*, a significance of P<0.05. †, reference group. OS, overall survival; PET, positron emission tomography; PET-P, PET positive; HR, hazard ratio; CI, confidence interval; ln, natural logarithm; WBMTV, whole-body metabolic tumor volume; WBTLG, whole-body total lesion glycolysis; chemo, chemotherapy; HE4, human epididymis protein 4; C-index, concordance index.
Survival curves using the Kaplan-Meier method were generated for the WBMTV, WBTLG, and post-PET treatment groups, which were determined according to median values (4.16 for WBMTV and 14.71 for WBTLG). The statistical evaluation indicated significant differences in OS among the subgroups (WBMTV and WBTLG: P<0.001; post-PET treatment: P=0.003) (Figure 3A-3C).

Differences in tumor markers between the PET-P and PET-N groups
In the PET-P group, the median serum CA125 and HE4 levels were 83.80 U/mL and 91.00 pmol/L, respectively, while in the PET-N group, they were 11.65 U/mL and 44.50 pmol/L, respectively. Between the PET-P and PET-N groups, there were statistically significant variations in the levels of serum CA125 and HE4 (P<0.001). The box plot in Figure 4A,4B shows the serum CA125 and HE4 distribution in the PET-P and PET-N groups.

Correlations between CA125, HE4, and 18F-FDG PET/CT parameters
Correlation analysis in the PET-P patient cohort revealed a weakly positive but statistically significant relationship between WBMTV, WBTLG, and serum HE4. Specifically, WBMTV correlated slightly more strongly with serum HE4 (r=0.337; P=0.007) (Figure 5A) than WBTLG did with HE4 (r=0.296; P=0.018) (Figure 5B). No statistically significant correlation was observed between CA125 and PET/CT parameters or between SUVmax and HE4 (P>0.05). In addition, the two tumor markers were significantly correlated (r=0.320; P=0.011) (Figure 5C).

The combined association of CA125, HE4, and PET findings on the survival of patients with EOC
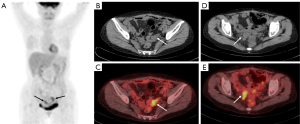
The HE4-P group had a PET positivity rate of 100%, while the CA125-P group had one of 94.1%. Patients were categorized into four groups based on the PET/CT, HE4, and CA125 test results. Given its 100% survival rate, the PET-N group was considered separately. The remaining three groups included PET-P, CA125-P and HE4-P; PET-P and HE4-P only or PET-P and CA125-P only; PET-P, HE4-N and CA125-N. The median survival time, 1-year survival rate, and 3-year survival rate for these four groups are presented in Table 5. The Kaplan-Meier OS curves, which combined posttreatment CA125, HE4, and PET/CT results (Figure 6), showed significant differences in the OS between these groups (P<0.001). Among the groups, patients testing positive on PET, CA125, and HE4 tests had the poorest OS prognosis, with 1- and 3-year survival rates of 68.8% and 37.5%, respectively. Representative 18F-FDG PET/CT images of one patient are shown in Figure 7.
Table 5
| Variable | 1-year survival rate (%) | 3-year survival rate (%) | Median survival (months) |
|---|---|---|---|
| PET-N | 100.0 | 100.0 | 76 |
| PET-P, HE4-P, CA125-P | 68.8 | 37.5 | 20 |
| PET-P, HE4-P, CA125-N; PET-P, HE4-N, CA125-P | 82.9 | 51.4 | 36 |
| PET-P, HE4-N, CA125-N | 91.7 | 50.0 | 40 |
CA125, carbohydrate antigen 125; HE4, human epididymis protein 4; PET, positron emission tomography; PET-N, PET negative; PET-P, PET positive; HE4-P, HE4 positive; CA125-P, CA125 positive; CA125-N, CA125 negative; HE4-N, HE4 negative.

Discussion
Studies have previously demonstrated the value of both posttreatment PET/CT and serum tumor markers, CA125 and HE4, in assessing survival prognosis in patients with OC (19-23); however, few studies have explored their interrelationships. Specifically, HE4, a tumor marker that has not yet been universally adopted in clinical practice, has substantial potential for further given its correlation with 18F-FDG PET/CT whole-body metabolic parameters. This study examined the relationships between these whole-body metabolic parameters, serum CA125 and HE4 concentrations, and OS in patients with EOC treated with surgery and chemotherapy. Furthermore, the prognostic value of the combined use of serum CA125, HE4, and PET/CT was ascertained.
The univariate analysis indicated there to be a significant difference in OS between the PET-N and PET-P groups on the first posttreatment PET/CT examination (HR =40.177, 95% CI: 2.690–600.134; P=0.007). It should be mentioned that the wider 95% CIs can be partly attributed to the case restrictions and the significant data differences between the PET-N and PET-P groups. These findings align with the prognostic investigation conducted by Han et al. (19) on ovarian malignancies. However, our research further clarifies the prognostic value of 18F-FDG PET/CT whole-body metabolic parameters. The univariate and multivariate analyses of the 63 patients cohort exhibiting abnormal 18F-FDG uptake indicated that volume-based metabolic parameters (lnWBMTV, lnWBTLG) as assessed on 18F-FDG PET/CT imaging can serve as independent risk factors for OS in patients with EOC. The WBSUVmax employed in this study merely represented the peak metabolic activity across all body lesions but not the extent of these lesions, which may explain its lack of significant association with OS. Conversely, WBMTV and WBTLG include volumetric attributes, with WBTLG also integrating both the volume of metabolically active lesions and the extent of glucose utilization within these lesions.
Regarding EOC, two prior studies explored the predictive significance of volumetric characteristics derived from postoperative 18F-FDG PET/CT. Liao et al. (24) analyzed 47 postoperative 18F-FDG PET/CT findings in patients with EOC. Their results showed that the higher the value of WBTLG is, the worse the clinical outcome and the shorter the survival time of the patients. WBTLG is a predictor of survival with statistical significance. Additionally, Liao et al. reported that WBMTV was not significantly associated with survival time after operation in patients with EOC. However, Gallicchio et al. (25) revealed that MTV was a better predictor of OS than was TLG in patients with EOC and peritoneal carcinoma, and the Cox proportional risk analysis showed that only MTV was correlated with OS. Our study demonstrated that WBMTV and WBTLG were remarkably associated with OS after treatment.
The discrepancies between the Liao et al. and Gallicchio et al. studies and our own seem to be chiefly related to the MTV and TLG measurement methods. The study by Liao et al. used a background method to segment systemic lesions to derive volume-based quantitative parameters, while Gallicchio et al. used a threshold of 42% of the SUVmax to segment peritoneal cancer lesions. Our study, on the other hand, used a threshold of 40% of the SUVmax. Furthermore, patients in our study received surgery combined with platinum-based chemotherapy before PET/CT examination, whereas in the studies by Liao et al. and Gallicchio et al., the prognostic value of postoperative, prechemotherapy PET/CT parameters was explored.
Post-PET therapy emerged as the sole independent prognostic indicator among the clinical variables evaluated in our study. In Model 1, which included lnWBMTV, patients who underwent secondary surgery combined with chemotherapy had a 0.393-fold higher mortality risk than did those who received no treatment or other forms of therapy. This risk was slightly lower at 0.367-fold in Model 2, which incorporated lnWBTLG. This suggests that a subsequent treatment strategy emphasizing the combination of surgery and chemotherapy could be the most effective approach for enhancing survival outcomes in patients diagnosed with EOC.
Based on the univariate analysis of all patients, we found a statistically significant difference in OS between the serum CA125 and HE4 positive and negative groups. In the PET-P patient cohort, these differences were insignificant, which may be partly due to the selection of subgroup thresholds and the overall poorer prognosis of PET-P patients.
This study compared CA125 and HE4 values between PET-P and PET-N patients, and the differences were found to be statistically significant (P<0.001), with CA125 and HE4 being significantly elevated in the PET-P group, which supports the clinical significance of performing PET/CT in patients with elevated CA125 and HE4.
Both tumor markers and 18F-FDG PET/CT can provide information about tumor burden, and the assessment of tumor burden is crucial for optimal patient management in clinical practice. However, there is little information in the literature regarding whether increases in serum tumor markers are connected to the spread of specific types of tumors (26). Our study showed a weak but statistically significant correlation between serum HE4 levels and systemic disease as assessed by WBMTV and WBTLG in the posttreatment PET-P patient cohort. Interestingly, serum CA125 failed to detect this correlation, which differed from the studies of Ye et al. (27). This discrepancy might be attributed to the differences in patient cohorts, as Ye et al.’s study only included patients diagnosed with the histological subtype of ovarian clear cell carcinoma. In conclusion, HE4 outperforms the traditional CA125 marker in predicting tumor burden. However, these findings should be verified through larger, multicenter studies.
There is little research examining the combination of serum CA125, HE4, and PET/CT. Sun et al. (28) analyzed 69 patients with OC suspected of tumor recurrence and metastasis after standard treatment, and the results indicated a sensitivity and specificity of the combined diagnosis of PET/CT and serum CA125 and HE4 of 100%. These results represent firm support for the clinical value of combining posttreatment serum CA125, HE4, and PET/CT for the diagnosis of recurrence and metastasis of OC. Our study showed that both the HE4-P and CA125-P groups had a high PET-P rate, and patients who were positive for CA125, HE4, and PET had a significantly lower OS and a markedly increased mortality risk after treatment. This suggests that the capacity of this combination to predict prognosis may help identify those patients with a poor prognosis, allowing for more aggressive treatment and attentive monitoring of these patients throughout clinical workups.
Our research does have some limitations that should be addressed. Primarily, given the retrospective study design, prospectively designed studies our needed to validate our findings. Additionally, the smaller sample sizes used in the subgroup analyses could have potentially underrepresented the value of FIGO staging and histological typing, as these were divided into only two subgroups. Finally, our threshold selection was fairly homogeneous, and the volume-based parameters did not differentiate between recurrence and metastasis locations. Given that the prognostic implications for patients can vary depending on the site of metastasis, the prognostic values of MTV and TLG may need to be further stratified based on their anatomical locations. Future research can explore the utility of combining more novel biomarkers with anatomical site-based volume metrics from PET/CT.
Conclusions
Posttreatment 18F-FDG PET/CT demonstrated significant prognostic value in predicting OS in patients with EOC. Posttreatment volume-based PET/CT parameters, WBMTV, and WBTLG were found to be independent risk factors for patients with EOC. Among clinical variables, therapy after PET was the only independent prognostic factor. Patients with positive indicators of CA125, HE4, and PET had significantly lower OS after treatment.
Acknowledgments
We appreciate the staff of the Department of Nuclear Medicine, First Hospital of Chongqing Medical University, for providing excellent technical assistance.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-859/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-859/coif). All authors have reported that this work was supported by the Health Commission Combined Science and Technology Project of Chongqing (No. 2020MSXM066). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No. K 2023-140). The requirement for informed consent was waived due to the retrospective nature of the design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Arora T, Mullangi S, Lekkala MR. Ovarian Cancer. StatPearls. Treasure Island (FL) ineligible companies. Copyright © 2023, StatPearls Publishing LLC.; 2023.
- Cabasag CJ, Arnold M, Rutherford M, Ferlay J, Bardot A, Morgan E, et al. Shifting incidence and survival of epithelial ovarian cancer (1995-2014): A SurvMark-2 study. Int J Cancer 2023;152:1763-77. [Crossref] [PubMed]
- Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I, Vergote I, Baert T, Belaroussi I, Dashora A, Olbrecht S, Planchamp F, Querleu D. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol 2019;30:672-705. [Crossref] [PubMed]
- Wang Q, Feng X, Liu X, Zhu S. Prognostic Value of Elevated Pre-treatment Serum CA-125 in Epithelial Ovarian Cancer: A Meta-Analysis. Front Oncol 2022;12:868061. [Crossref] [PubMed]
- Chan JK, Tian C, Kesterson JP, Richardson MT, Lin K, Tewari KS, Herzog T, Kapp DS, Monk BJ, Casablanca Y, Hanjani P, Wenham RM, Walker J, McNally L, Copeland LJ, Robertson S, Lentz S, Spirtos NM, Bell JG. The clinical and prognostic significance of pre-chemotherapy serum CA-125 in high-risk early stage ovarian cancer: An NRG/GOG ancillary study. Gynecol Oncol 2022;167:429-35. [Crossref] [PubMed]
- Li Z, Yin H, Ren M, Shen Y. Prognostic Significance of CA125 Dynamic Change for Progression Free Survival in Patients with Epithelial Ovarian Carcinoma. Med Sci Monit 2020;26:e925051. [Crossref] [PubMed]
- Timmermans M, Zwakman N, Sonke GS, Van de Vijver KK, Duk MJ, van der Aa MA, Kruitwagen RF. Perioperative change in CA125 is an independent prognostic factor for improved clinical outcome in advanced ovarian cancer. Eur J Obstet Gynecol Reprod Biol 2019;240:364-9. [Crossref] [PubMed]
- Plotti F, Guzzo F, Schirò T, Terranova C, De Cicco Nardone C, Montera R, Luvero D, Scaletta G, Lopez S, Capriglione S, Benedetti Panici P, Angioli R. Role of human epididymis protein 4 (HE4) in detecting recurrence in CA125 negative ovarian cancer patients. Int J Gynecol Cancer 2019; Epub ahead of print. [Crossref]
- Kim B, Park Y, Kim B, Ahn HJ, Lee KA, Chung JE, Han SW. Diagnostic performance of CA 125, HE4, and risk of Ovarian Malignancy Algorithm for ovarian cancer. J Clin Lab Anal 2019;33:e22624. [Crossref] [PubMed]
- Sun ML, Yang ZY, Wu QJ, Li YZ, Li XY, Liu FH, Wei YF, Wen ZY, Lin B, Gong TT. The Role of Human Epididymis Protein 4 in the Diagnosis and Prognosis of Diseases: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. Front Med (Lausanne) 2022;9:842002. [Crossref] [PubMed]
- Lee S, Choi S, Lee Y, Chung D, Hong S, Park N. Role of human epididymis protein 4 in chemoresistance and prognosis of epithelial ovarian cancer. J Obstet Gynaecol Res 2017;43:220-7. [Crossref] [PubMed]
- Delgado Bolton RC, Aide N, Colletti PM, Ferrero A, Paez D, Skanjeti A, Giammarile F. EANM guideline on the role of 2-[18F]FDG PET/CT in diagnosis, staging, prognostic value, therapy assessment and restaging of ovarian cancer, endorsed by the American College of Nuclear Medicine (ACNM), the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the International Atomic Energy Agency (IAEA). Eur J Nucl Med Mol Imaging 2021;48:3286-302.
- Zhang C, Liao C, Penney BC, Appelbaum DE, Simon CA, Pu Y. Relationship between Overall Survival of Patients with Non-Small Cell Lung Cancer and Whole-Body Metabolic Tumor Burden Seen on Postsurgical Fluorodeoxyglucose PET Images. Radiology 2015;275:862-9. [Crossref] [PubMed]
- Gadducci A, Simonetti E, Guidoccio F, Manca G, Giorgetti A, Depalo T, Cosio S, Miccoli M, Volterrani D. Have Volume-based Parameters of Positron Emission Tomography/Computed Tomography Prognostic Relevance for Patients With Potentially Platinum-responsive Recurrent Ovarian Cancer? A Single Center Italian Study. Anticancer Res 2021;41:1937-44. [Crossref] [PubMed]
- Sun G, Cheng C, Li X, Wang T, Yang J, Li D. Metabolic tumor burden on postsurgical PET/CT predicts survival of patients with gastric cancer. Cancer Imaging 2019;19:18. [Crossref] [PubMed]
- Lee JW, Cho A, Lee JH, Yun M, Lee JD, Kim YT, Kang WJ. The role of metabolic tumor volume and total lesion glycolysis on 18F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur J Nucl Med Mol Imaging 2014;41:1898-906. [Crossref] [PubMed]
- Han EJ, Park HL, Lee YS, Park EK, Song MJ, Yoo IR, Kim SH, Choi WH. Clinical usefulness of post-treatment FDG PET/CT in patients with ovarian malignancy. Ann Nucl Med 2016;30:600-7. [Crossref] [PubMed]
- Hebel CB, Behrendt FF, Heinzel A, Krohn T, Mottaghy FM, Bauerschlag DO, Verburg FA. Negative 18F-2-fluorodeoxyglucose PET/CT predicts good cancer specific survival in patients with a suspicion of recurrent ovarian cancer. Eur J Radiol 2014;83:463-7. [Crossref] [PubMed]
- Chu LC, Tsai HL, Wang H, Crandall J, Javadi MS, Wahl RL. Posttreatment FDG PET/CT in predicting survival of patients with ovarian carcinoma. EJNMMI Res 2016;6:42. [Crossref] [PubMed]
- Braicu EI, Chekerov R, Richter R, Pop C, Nassir M, Loefgren H, Stamatian F, Muallem MZ, Hall C, Fotopoulou C, Sehouli J, Pietzner K. HE4 expression in plasma correlates with surgical outcome and overall survival in patients with first ovarian cancer relapse. Ann Surg Oncol 2014;21:955-62. [Crossref] [PubMed]
- Salminen L, Gidwani K, Grènman S, Carpén O, Hietanen S, Pettersson K, Huhtinen K, Hynninen J. HE4 in the evaluation of tumor load and prognostic stratification of high grade serous ovarian carcinoma. Acta Oncol 2020;59:1461-8. [Crossref] [PubMed]
- Liao S, Lan X, Cao G, Yuan H, Zhang Y. Prognostic predictive value of total lesion glycolysis from 18F-FDG PET/CT in post-surgical patients with epithelial ovarian cancer. Clin Nucl Med 2013;38:715-20. [Crossref] [PubMed]
- Gallicchio R, Nardelli A, Venetucci A, Capacchione D, Pelagalli A, Sirignano C, Mainenti P, Pedicini P, Guglielmi G, Storto G. F-18 FDG PET/CT metabolic tumor volume predicts overall survival in patients with disseminated epithelial ovarian cancer. Eur J Radiol 2017;93:107-13. [Crossref] [PubMed]
- Glickman A, Paredes P, Carreras-Diéguez N, Niñerola-Baizán A, Gaba L, Pahisa J, Fusté P, Del Pino M, Díaz-Feijóo B, González-Bosquet E, Agustí N, Sánchez-Izquierdo N, Fuster D, Perissinotti A, Romero I, Fernández-Galán E, Carrasco JL, Gil-Ibáñez B, Torné A. Evaluation of patients with advanced epithelial ovarian cancer before primary treatment: correlation between tumour burden assessed by [18F]FDG PET/CT volumetric parameters and tumour markers HE4 and CA125. Eur Radiol 2022;32:2200-8.
- Ye S, Liu S, Zhou S, Xiang L, Wu X, Yang H. The role of 18F-FDG PET/CT-based quantitative metabolic parameters in patients with ovarian clear cell carcinoma. Cancer Biomark 2020;27:189-94. [Crossref] [PubMed]
- Sun J, Cui XW, Li YS, Wang SY, Yin Q, Wang XN, Gu L. The value of 18F-FDG PET/CT imaging combined with detection of CA125 and HE4 in the diagnosis of recurrence and metastasis of ovarian cancer. Eur Rev Med Pharmacol Sci 2020;24:7276-83. [Crossref] [PubMed]


