
Cardiac magnetic resonance analysis of left atrium function in patients with pre-apical hypertrophic cardiomyopathy
Introduction
In clinical practice, it is not uncommon to observe patients presenting with unexplained T wave inversion on electrocardiogram (ECG) combined with thickened left ventricular (LV) apex but less than 15 mm. These patients had been proposed as a preclinical scope of apical hypertrophy cardiomyopathy (pre-ApHCM) (1,2). However, the relatively limited studies just focused on the left ventricle structure instead of the changes of global or regional heart function of these patients, especially for left atrial (LA) function (1,3).
Cardiac hypertrophy could enlarge LA, which is a risk factor of atrial fibrillation (AF) in patients with ApHCM (4). Cardiac hypertrophy could also alter the LA function (5-7), which acts as an integral part of global cardiac function (8). However, how the left atrium structure (volumetric parameters) and function (deformation parameters) change in the pre-ApHCM patients still remains unknown.
With the advancement of cardiac magnetic resonance (CMR) technology, it is now able to assess atrial function, in particular strain imaging, thereby enabling the evaluation of LA reservoir (in systole), conduit and contractile function (in diastole) (9). A previous study has found that pre-ApHCM patients had abnormal LV apical morphology (1). And more than 70% of these patients have more than 15% increase of LV apical wall thickness including one-fifth progression to typical ApHCM during more than 2-year follow-up (3). However, the LA function of this kind of patient has not been well explored. In this study we used CMR to evaluate the LA function in pre-ApHCM patients and compared it with patients with ApHCM. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-466/rc).
Methods
Study population
In this retrospective study, a total of 3,593 CMR reports from April 2016 to December 2021 were reviewed in the Radiology Department of Beijing Anzhen Hospital, China. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Beijing Anzhen hospital and individual consent for this retrospective analysis was waived.
The inclusion criteria of ApHCM included: (I) deep giant T-wave inversion on 12-lead ECG (10) (II) end-diastolic left ventricle apical wall thickness ≥15 mm based on CMR. The inclusion criteria of the pre-ApHCM were as follows: (I) deep giant T-wave inversion (negative T-wave voltage of ≥5 mm) on 12-lead ECG, at least 3 contiguous leads (most prominent inV3–V leads); (II) no ApHCM on echocardiography, (III) the end-diastolic LV apical wall thickness <15 mm but thicker than the basal segment confirmed with apical 2 and 4 chamber CMR views (1-3).
Patients were excluded if they had (I) coronary artery disease; (II) arrhythmia, include bundle branch block, AF; (III) hypertension with or without medical control; (IV) severe valvular disease, (V) pericardial disease, (VI) cardiac tumor, (VII) systemic disease involving heart; and (VIII) history of cardiac surgery.
We finally identified 31 pre-ApHCM patients in this study. Furthermore, 40 ApHCM and 31 normal controls were included for comparison.
All the normal controls did not have any history of cardiovascular disease, with normal physical examination and normal ECG, echocardiography and CMR.
CMR data acquisition
All CMR studies were performed on a 3.0 T Siemens scanner (MagnetomVerio; Siemens AG Healthcare, Erlangen, Germany) with retrospective ECG gating and 32-channel phased-array coil. Standardized imaging protocol were performed consisting of steady state free precession breath-hold cine images and late gadolinium enhancement (LGE) images (11,12). The whole ventricles from the annulus of the atrioventricular valves to the apex were covered in contiguous short axis stack cines, with 25 phases per cardiac cycle and long axis planes (2-, 4-, and 3-chamber views) using retrospective ECG gating true fast imaging with steady precession (True FISP) cine sequence were taken. LGE images, 10 minutes after a 0.2 mmol/kg intravenous dose of Gd-DTPA (Magnevist, Bayer Schering, Germany, 0.2 mmol/kg) were taken with a prospectively ECG-gated gradient echo sequence with an inversion prepulse during breath hold in a series of short-axis planes and a 4- and 2-chamber long-axis plane. Phase-sensitive inversion-recovery was used to obviate the need for a precise setting of the TI. Imaging acquisition parameters were listed as follows: repetition time/echo time, 4.1/1.6 ms; flip angle, 20°; image matrix, 256×130; section thickness, 8 mm (contiguous short axis) or 5 mm (long axis images), with no intersection gap.
CMR image analysis
The analyses of LV function, masses, LV wall thickness were performed on a commercially available workstation CVI42 software (Version 5.6.3 Circle Cardiovascular Imaging, Calgary, Canada). Short-axis cine images and long axis cine views were used for semi-automated analysis. Endocardial and epicardial borders were identified automatically and amended by a radiologist (with 10 years’ experience in cardiac MR image interpretation) on the short-axis cine images. Papillary muscles were excluded from volumes and the right ventricular insertion sites were marked to indicate the outer border of the antero-septum and infra-septum. LV range was marked on 2-chamber or 4-chamber images. Left ventricular ejection fraction (LVEF) was computed automatically after the endocardial border was drawn. Standard apical 4- and 2-chamber views at end-diastole were used to obtained the maximal apical wall thickness.
LA feature tracking
LA volume and function were performed on dedicated CMR post-processing software (QStrain, Medis Suite 3.1, Leiden, the Netherlands). A point-and-click approach was used to trace the LA endocardial boarder in both the 2- and 4-chamber views. Pulmonary veins and LA appendage were excluded carefully. Then the contour was detected automatically throughout the entire stack. The CMR-FT was verified and readjusted by the operator (H.W., with >10 years of experience in interpreting CMR) to ensure accurate tracking.
The end-diastolic and end-systolic phases were adjusted manually if necessary. LA global strain was calculated as the average of the 2- and 4-chamber views (7,13). On each strain curve (Figure 1), LA endocardial longitudinal strain parameters, including εs (reservoir phase strain, corresponding to LA reservoir function), εa, (LA contraction phase strain, corresponding to LA booster pump function) and εe (conduit phase strain, corresponding to LA conduit function, εe = εs − εa), were analyzed (7,9,14,15). Correspondingly, on LA volume curve (Figure 2), LA volumetric parameters, including LA maximal (LA Vmax, at the LV end-systole), pre-atrial contractile (LA Vprac, at the LV diastole before LA contraction) and minimal volume (LA Vmin, at the late LV end-diastole after LA contraction), were obtained (7,15,16). LA reservoir, passive and booster EF were calculated from LA volumes according to the following equations (7,17):


Statistical analysis
Data were analyzed using SPSS software version 24.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables with normal distribution were expressed as mean ± standard deviation; two-tailed one-way ANOVA was used to analyze the difference of three groups (ApHCM, pre-ApHCM and normal control groups). Pearson correlation test was used for correlation analysis. P values of <0.05 were regarded as statistically significant.
Results
Of 43 ApHCM patients, three were excluded due to hypertension. Ten pre-ApHCM patients were excluded: three due to hypertension, two due to coronary artery disease, one due to atrial septal defect, two due to blurred ECG and two due to poor CMR imaging quality). Finally, a total of 102 cases were included consisting of 40 cases of ApHCM, 31 cases of pre-ApHCM, and 31 cases of normal control (Figure 3).

Table 1 shows the main baseline characteristics of these three groups. Both the ApHCM (68.77%±7.09%) and pre-ApHCM (69.50%±7.22%) had higher LVEF than the control group (64.37%±6.26%), although there was no significant difference between pre-ApHCM and ApHCM. The thickness of diastolic LV apex (ApHCM: 19.36±3.05 mm, pre-ApHCM: 11.92±1.37 mm, control group: 5.70±1.09 mm) as well as the myocardial mass of LV during diastole (ApHCM: 134.96±41.06 g, pre-ApHCM: 111.44±27.34 g, control group: 83.99±17.96 g) increased progressively from normal to ApHCM with pre-ApHCM having an intermediate value, with significant differences among the three groups (P<0.05). Among the 40 ApHCM patients, 31 were injected with contrast media, and 23 had LGE. Twenty-eight out of the 31 pre-ApHCM patients were injected with contrast media, five patients had LGE, with a significant difference between the two groups (P<0.05). No adverse reactions were recorded.
Table 1
| Variable | Groups | P value | |||||
|---|---|---|---|---|---|---|---|
| ApHCM (N=40) | pre-ApHCM (N=31) | Control group (N=31) | ApHCM vs. pre-ApHCM | ApHCM vs. control group | pre-ApHCM vs. control group | ||
| Age (years) | 54.13±15.00 | 49.16±13.92 | 48.06±10.95 | 0.129 | 0.065 | 0.751 | |
| LVEF (%) | 68.77±7.09 | 69.50±7.22 | 64.37±6.26 | 0.66 | 0.009 | 0.004 | |
| LV mass (g) | 134.96±41.06 | 111.44±27.34 | 83.99±17.96 | 0.002 | <0.001 | 0.001 | |
| LGE (Ncontrast, NLGE, %) | (31, 23, 74.19%) | (28, 5, 17.86%) | – | <0.001 | – | – | |
| Thickness of diastolic LV apex (mm) | 19.36±3.05 | 11.92±1.37 | 5.70±1.09 | <0.001 | <0.001 | <0.001 | |
Data are presented as mean ± standard deviation or n (%). There was no difference in age among the three groups. In terms of LVEF, both the ApHCM and pre-ApHCM have higher LVEF than the control group although there was no significant difference between pre-ApHCM and ApHCM. The thickness of diastolic LV apex as well as the myocardial mass of LV during diastole increased progressively from normal to ApHCM with pre-ApHCM having an intermediate value. ApHCM, apical hypertrophy cardiomyopathy; pre-ApHCM, preclinical scope of ApHCM; LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement.
Table 2 shows that there was weak or no correlation between the LV mass and LA deformation parameters in both ApHCM group and pre-ApHCM group.
Table 2
| Variable | Pearson correlation | ||
|---|---|---|---|
| LAεs | LAεa | LAεe | |
| LV mass (ApHCM) | −0.25 | −0.32 | 0.04 |
| LV mass (pre-ApHCM) | 0.03 | 0.12 | 0.05 |
There was weak or no correlation between the LV mass and LA deformation parameters in both ApHCM group and pre-ApHCM group. LA, left atrium; εs, LA reservoir phase strain, corresponding to LA reservoir function; εa, LA contraction phase strain, corresponding to LA booster pump function; εe, LA conduit phase strain, corresponding to LA conduit function; LV, left ventricle; ApHCM, apical hypertrophy cardiomyopathy; pre-ApHCM, preclinical scope of ApHCM.
LA volumetric, LGE and deformation parameters
Table 3 shows that all LA volumetric parameters (LA Vmax, LA Vmin, LA Vprac) in both ApHCM group and pre-ApHCM group were significantly higher than that in the control group (P=0.004, <0.001, <0.001 respectively), while there was no difference between the ApHCM and pre-ApHCM (P=0.232, 0.051, 0.085 respectively). LA Vmax in ApHCM, pre-ApHCM and control group were 79.67±28.69, 72.32±27.39 and 58.80±18.13 mL respectively. The LA Vmin in the three groups were 43.73±23.83, 35.05±17.27, and 23.48±8.98 mL respectively, while the LA Vprac were 64.91±24.30, 56.11±22.15, 42.51±14.67 mL.
Table 3
| Variable | Groups | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| ApHCM (N=40) | pre-ApHCM (N=31) | Control group (N=31) | ApHCM vs. pre-ApHCM vs. control group | ApHCM vs. pre-ApHCM | ApHCM vs. control group | pre-ApHCM vs. control group | ||
| LA volumetric parameters | ||||||||
| LA Vmax (mL) | 79.67±28.69 | 72.32±27.39 | 58.80±18.13 | 0.004 | 0.232 | 0.001 | 0.040 | |
| LA Vmin (mL) | 43.73±23.83 | 35.05±17.27 | 23.48±8.98 | <0.001 | 0.051 | <0.001 | 0.015 | |
| LA Vprac (mL) | 64.91±24.30 | 56.11±22.15 | 42.51±14.67 | <0.001 | 0.085 | <0.001 | 0.013 | |
| LA reservoir function | ||||||||
| LA total EF (%) | 46.63±11.50 | 51.94±10.53 | 60.27±7.7 | <0.001 | 0.031 | <0.001 | 0.002 | |
| LAεs | 37.11±12.40 | 44.43±12.93 | 54.45±14.46 | <0.001 | 0.023 | <0.001 | 0.004 | |
| LA booster function | ||||||||
| LA booster EF (%) | 34.36±16.24 | 37.97±12.38 | 44.45±10.87 | 0.01 | 0.272 | 0.003 | 0.064 | |
| LAεa | 22.91±8.63 | 24.28±7.95 | 29.75±9.81 | 0.005 | 0.518 | 0.002 | 0.016 | |
| LA conduit function | ||||||||
| LA passive EF (%) | 17.82±8.9 | 22.17±9.7 | 28.06±7.4 | <0.001 | 0.041 | <0.001 | 0.010 | |
| LAεe | 9.80±4.69 | 12.73±5.84 | 17.23±6.03 | <0.001 | 0.028 | <0.001 | 0.002 | |
Data are presented as mean ± standard deviation. There were no differences in all volumetric and LA booster function parameters between the ApHCM and pre-ApHCM group. All the volumetric parameters in pre-ApHCM group were higher than that in the control group and all the LA function parameters except LA booster EF in pre-ApHCM group were impaired. The LA reservoir as well as conduit function in pre-ApHCM group were significantly impaired compared to the normal controls but higher than the ApHCM, i.e., occupying an intermediate position. ApHCM, apical hypertrophy cardiomyopathy; pre-ApHCM, preclinical scope of ApHCM; LA Vmax, maximal volume of left atrium; LA Vmin, minimal volume of left atrium; LA Vprac, pre-atrial contractile of left atrium; LVEF, left ventricular ejection fraction; εs, LA reservoir phase strain, corresponding to LA reservoir function; εa, LA contraction phase strain, corresponding to LA booster pump function; εe, LA conduit phase strain, corresponding to LA conduit function.
LA reservoir (LA total EF, εs) function parameters were significantly different among the three groups (both P<0.001), with the ApHCM group (46.63%±11.50%, 37.11±12.40) having the lowest. In contrast, these parameters were intermediate in the pre-ApHCM group (51.94%±10.53%, 44.43±12.93), and the highest in the control group (60.27%±7.7%, 54.45±14.46).
LA conduit function (LA passive EF, εe) parameters, same as the LA reservoir function, were also significantly different among the three groups (both P<0.001), with the ApHCM group (17.82%±8.9%, 9.80±4.69) having the lowest, intermediate in the pre-ApHCM group (22.17%±9.7%, 12.73±5.84) were, and the highest in the control group (28.06%±7.4%, 17.23±6.03).
When compared with the control group (29.75±9.81), one of the LA booster pump function marks (εa) was significantly impaired in both the ApHCM (22.91±8.63) (P=0.002) and pre-ApHCM groups (24.28±7.95) (P=0.016). While another LA booster pump function mark (LA booster EF), compared with the control group (44.45%±10.87%), the pre-ApHCM (37.97%±12.38%) showed no difference (P=0.064) while in the ApHCM group (34.36%±16.24%) was significant impaired (P=0.003). Neither εa nor the LA booster EF show difference between the ApHCM group and pre-ApHCM group (P=0.272, 0.518. respectively) (Table 3).
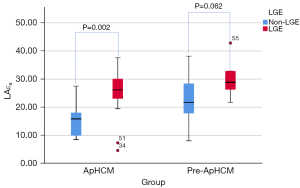
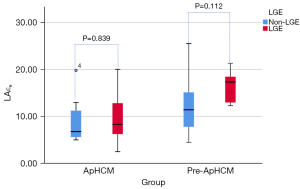
Figures 4-6 show that in ApHCM group, both LAεs (40.49±11.47 vs. 28.35±11.72) and LAεa (25.86±8.05 vs. 15.45±6.25) were significantly higher in patients with LGE than without LGE (P=0.016, 0.002, respectively). LAεe shows no difference between patients with LGE than without LGE (P=0.839). None of the LA strain parameters have difference between patients with LGE than without LGE in pre-ApHCM group (LAεs, P=0.128; LAεa, P=0.062; LAεe, P=0.112).

Discussion
In this study we compared the LA structure (volumetric parameters) and function (deformation parameters) among ApHCM and pre-ApHCM and normal controls by using CMR feature tracking. Compared with the control group, the ApHCM group had larger LA volumetric and impaired LA function parameters. All the volumetric parameters in the pre-ApHCM group were higher than those in the control group and all the LA function parameters but the LA booster EF in pre-ApHCM group were impaired. While there were no differences in all volumetric and LA booster function parameters between the ApHCM and pre-ApHCM group. The LA reservoir as well as conduit function, however, in pre-ApHCM group were significantly impaired compared to the normal controls but higher than the ApHCM, i.e., occupying an intermediate position. LGE seems to be associated to both LA reservoir and booster function.
Generally, patients with apical wall thickness ≥15 mm at the end diastolic phase are diagnosed as ApHCM, based on the guidelines recommended by ESC (18). However, many researchers realized that the 15 mm standard was too restrictive. A number of studies confirmed that even if the apex of myocardial thickness is less than 15 mm, based on the apical and basal wall thickness ratio or the morphology of the apex, combined with unexplainable obvious T-wave inversion in 3 contiguous leads (most prominent inV3–V leads), then ApHCM could be diagnosed, or at least a preclinical stage of ApHCM (1,2,19-22).
To our knowledge, none of the previous studies have focused on the functional changes of the LA in pre-ApHCM patients. Hypertrophy of the left ventricle is associated with left ventricular diastolic dysfunction especially in HCM (23-25), which will cause an elevation of LV filling pressures and left atrium afterload (26,27). As the LV wall thickness increased, the LV diastolic function, which can be categorized by the LA strain, decreased (28). Our study was conducted to address this gap in the current literature.
Our results indicated that reservoir and conduit functions are significantly affected by increased LV apex wall thickness. To some extent, it can be seen as “mirrors” LV apex thickening and dysfunction process (29,30). Reservoir and conduit functions significantly decrease, with pre-ApHCM group being significantly impaired compared to the normal controls but higher than the ApHCM. While the LA booster function was not. LAεa, which represents booster function, was impaired in both ApHCM and pre-ApHCM groups compared with control group, while there was no difference between the ApHACM and pre-ApHCM group. A previous study suggests that LA booster function was independent of LV function (31). Our results are consistent with these reports. Before LV apex reached the ApHCM standard (15 mm), when LV filling pressures increased significantly, the limits of LA preload reserve were reached, LA booster function would not decrease with the LV apex thickening, then the LA would behave predominantly as a conduit (28,32). Although our results show no difference in the LA booster EF between the pre-ApHCM and normal controls, there was a significant trend that LA booster EF in pre-ApHCM decreased.
All LA volumetric parameters, different from the reservoir function and conduit function, in the pre-ApHCM were not different from the ApHCM but significantly larger than normal controls. It indicates that LA strain has greater sensitivity in detecting early pathologic changes in LA function before the LA volumetric change, because the LA may take time to remodel.
Limitations
First, this was a single-center, retrospective study with a small group of patients. Second, no follow-up data was available. Third, we did not use the LA and LV volume indexes as we could not obtain all the body surface area information from this retrospective study. Fourth, the genetic testing was not performed in this study, which would have interesting results. These limitations could be addressed by future studies with inclusion of more cases.
Conclusions
This study shows that the LA function in the patients with unexplainable giant T-wave inversion and apical thickness ≤15 mm had similar LA function features to typical ApHCM but significantly different from normal controls. In pre-ApHCM and ApHCM patients, the LA reservoir and conduit function impaired earlier before the left atrium enlarged and decreased progressively as apex wall thickens, while the booster function independently decreased in both pre-ApHCM and ApHCM patients. These findings may help us to understand the LA functional change from pre-ApHCM to ApHCM, and to detect subclinical changes in patients with pre-ApHCM before overt hypertrophy or clinical symptoms develop.
Acknowledgments
The abstract of this study was published in the Great Wall International Congress of Cardiology 2020/Asian Heart Society Congress 2020.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-466/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-466/coif). Z.S. serves as an unpaid associate editor of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Beijing Anzhen hospital and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu B, Lu M, Zhang Y, Song B, Ling J, Huang J, Yin G, Lan T, Dai L, Song L, Jiang Y, Wang H, He Z, Lee J, Yong HS, Patel MB, Zhao S. CMR assessment of the left ventricle apical morphology in subjects with unexplainable giant T-wave inversion and without apical wall thickness ≥15 mm. Eur Heart J Cardiovasc Imaging 2017;18:186-94. [Crossref] [PubMed]
- Flett AS, Maestrini V, Milliken D, Fontana M, Treibel TA, Harb R, Sado DM, Quarta G, Herrey A, Sneddon J, Elliott P, McKenna W, Moon JC. Diagnosis of apical hypertrophic cardiomyopathy: T-wave inversion and relative but not absolute apical left ventricular hypertrophy. Int J Cardiol 2015;183:143-8. [Crossref] [PubMed]
- Li S, He J, Xu J, Zhuang B, Wu B, Wei B, Huang J, Yin G, Chen X, Zhu Z, Wang H, Zhao S, Lu M. Patients who do not fulfill criteria for hypertrophic cardiomyopathy but have unexplained giant T-wave inversion: a cardiovascular magnetic resonance mid-term follow-up study. J Cardiovasc Magn Reson 2021;23:67. [Crossref] [PubMed]
- Lee SE, Park JK, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B. Impact of atrial fibrillation on the clinical course of apical hypertrophic cardiomyopathy. Heart 2017;103:1496-501. [Crossref] [PubMed]
- Debonnaire P, Joyce E, Hiemstra Y, Mertens BJ, Atsma DE, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Left Atrial Size and Function in Hypertrophic Cardiomyopathy Patients and Risk of New-Onset Atrial Fibrillation. Circ Arrhythm Electrophysiol 2017;10:e004052. [Crossref] [PubMed]
- Hinojar R, Zamorano JL, Fernández-Méndez M, Esteban A, Plaza-Martin M, González-Gómez A, Carbonell A, Rincón LM, Nácher JJJ, Fernández-Golfín C. Prognostic value of left atrial function by cardiovascular magnetic resonance feature tracking in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 2019;35:1055-65. [Crossref] [PubMed]
- Yang Y, Yin G, Jiang Y, Song L, Zhao S, Lu M. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson 2020;22:1. [Crossref] [PubMed]
- Yang CH, Liu HT, Lee HL, Lin FC, Chou CC. Left atrial booster-pump function as a predictive parameter for atrial fibrillation in patients with severely dilated left atrium. Quant Imaging Med Surg 2022;12:2523-34. [Crossref] [PubMed]
- Gan GCH, Ferkh A, Boyd A, Thomas L. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther 2018;8:29-46. [Crossref] [PubMed]
- Kang S, Choi WH. Pseudonormalization of negative T wave during stress test in asymptomatic patients without ischemic heart disease: a clue to apical hypertrophic cardiomyopathy? Cardiology 2013;124:91-6. [Crossref] [PubMed]
- Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel ESociety for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013;15:91. [Crossref] [PubMed]
- Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel ESociety for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 2008;10:35. [Crossref] [PubMed]
- Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson 2018;20:71. [Crossref] [PubMed]
- Evin M, Redheuil A, Hatem S, Rosenbaum D, Bouazizi-Verdier K, De Cesare A, Cluzel P, Kachenoura N. Left atrium wall tracking from MR images for strain assessment. Comput Methods Biomech Biomed Engin 2014;17:14-5. [Crossref] [PubMed]
- Li L, Chen X, Yin G, Yan W, Cui C, Cheng H, Lu M, Zhao S. Early detection of left atrial dysfunction assessed by CMR feature tracking in hypertensive patients. Eur Radiol 2020;30:702-11. [Crossref] [PubMed]
- Chen X, Pan J, Shu J, Zhang X, Ye L, Chen L, Hu Y, Yu R. Prognostic value of regional strain by cardiovascular magnetic resonance feature tracking in hypertrophic cardiomyopathy. Quant Imaging Med Surg 2022;12:627-41. [Crossref] [PubMed]
- Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, Sohns JM, Staab W, Bettencourt N, Unterberg-Buchwald C, Hasenfuß G, Lotz J, Schuster A. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson 2014;16:60. [Crossref] [PubMed]
- Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79.
- Klarich KW, Attenhofer Jost CH, Binder J, Connolly HM, Scott CG, Freeman WK, Ackerman MJ, Nishimura RA, Tajik AJ, Ommen SR. Risk of death in long-term follow-up of patients with apical hypertrophic cardiomyopathy. Am J Cardiol 2013;111:1784-91. [Crossref] [PubMed]
- Suzuki J, Shimamoto R, Nishikawa J, Yamazaki T, Tsuji T, Nakamura F, Shin WS, Nakajima T, Toyo-Oka T, Ohotomo K. Morphological onset and early diagnosis in apical hypertrophic cardiomyopathy: a long term analysis with nuclear magnetic resonance imaging. J Am Coll Cardiol 1999;33:146-51. [Crossref] [PubMed]
- Fattori R, Biagini E, Lorenzini M, Buttazzi K, Lovato L, Rapezzi C. Significance of magnetic resonance imaging in apical hypertrophic cardiomyopathy. Am J Cardiol 2010;105:1592-6. [Crossref] [PubMed]
- Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004;90:645-9. [Crossref] [PubMed]
- Chacko BR, Karur GR, Connelly KA, Yan RT, Kirpalani A, Wald R, Jimenez-Juan L, Jacob JR, Deva DP, Yan AT. Left ventricular structure and diastolic function by cardiac magnetic resonance imaging in hypertrophic cardiomyopathy. Indian Heart J 2018;70:75-81. [Crossref] [PubMed]
- Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 2017;121:749-70. [Crossref] [PubMed]
- Sanderson JE, Gibson DG, Brown DJ, Goodwin JF. Left ventricular filling in hypertrophic cardiomyopathy. An angiographic study. Br Heart J 1977;39:661-70. [Crossref] [PubMed]
- Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TS. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 2011;12:421-30. [Crossref] [PubMed]
- Stefanadis C, Dernellis J, Toutouzas P. A clinical appraisal of left atrial function. Eur Heart J 2001;22:22-36. [Crossref] [PubMed]
- Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc Imaging 2017;10:735-43. [Crossref] [PubMed]
- Singh A, Medvedofsky D, Mediratta A, Balaney B, Kruse E, Ciszek B, Shah AP, Blair JE, Maffessanti F, Addetia K, Mor-Avi V, Lang RM. Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int J Cardiovasc Imaging 2019;35:23-32. [Crossref] [PubMed]
- Kao YC, Hung MJ. Echocardiographic Evaluation of Left Atrial Function to Discriminate Non-Valvular Atrial Fibrillation Development in Patients with Apical Hypertrophic Cardiomyopathy. Acta Cardiol Sin 2020;36:33-43. [Crossref] [PubMed]
- Ramkumar S, Yang H, Wang Y, Nolan M, Negishi T, Negishi K, Marwick TH. Association of the Active and Passive Components of Left Atrial Deformation with Left Ventricular Function. J Am Soc Echocardiogr 2017;30:659-66. [Crossref] [PubMed]
- Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:1961-77. [Crossref] [PubMed]


