
Imaging findings of posterior fossa subdural empyema secondary to neonatal bacterial meningitis
Introduction
Subdural empyema is a life-threatening condition that refers to the accumulation of purulent material between the dura and the arachnoid mater in the cranial cavity (1). Subdural empyema in the posterior fossa is an extremely rare complication of neonatal bacterial meningitis (2). Due to the lack of specific clinical symptoms and indicators, early and accurate diagnosis is challenging (3). In this report, we present the imaging manifestations of 2 cases with posterior fossa subdural empyema secondary to neonatal bacterial meningitis to improve its recognition and diagnostic level.
Cases preparation
Case 1
A 6-day-old boy presented to the hospital with repeated fever. Laboratory tests revealed an elevated cerebrospinal fluid (CSF) white blood cell count (1,757×106/L), a decreased CSF glucose level (0.45 mmol/L) as well as an elevated CSF protein concentration (3,405 mg/L). The diagnosis of neonatal bacterial meningitis was confirmed by positive CSF culture of Escherichia coli. He underwent a magnetic resonance imaging (MRI) examination on the 22nd day after birth (Figure 1A-1J), and bilateral lesions were detected in the posterior fossa with hyperintense areas on T2-weighted imaging (T2WI) (Figure 1B) and fluid-attenuated inversion recovery (FLAIR) (Figure 1C) as well as restricted diffusion (Figure 1D-1E). The margin of the lesion was hyperintense on T1-weighted imaging (T1WI) (Figure 1A) and significantly enhanced (Figure 1F). The left transverse sinus was not shown on the reconstruction magnetic resonance venography (MRV) image (Figure 1G) but could be found on the original image (Figure 1H), which was more slender than on the right side. MRI findings confirmed the presence of infratentorial subdural empyema. The lesion was shown to have been absorbed on a follow-up magnetic resonance (MR) exam performed on the 46th day [Figure 1I: diffusion-weighted imaging (DWI); Figure 1J: T1WI]. After admission, he received a combination of intravenous meropenem and amoxicillin for anti-infection treatment. About 8 weeks later, he was discharged from hospital when his infection indicators had returned to normal levels.
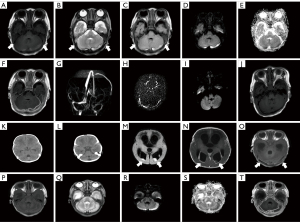
Case 2
An 8-day-old girl was admitted to the hospital due to intermittent fever. Laboratory tests showed an elevated CSF white blood cell count (70×106/L), a decreased CSF glucose level (0.85 mmol/L) as well as an elevated CSF protein concentration (12,719 mg/L). The culture of the CSF grew Escherichia coli. No obvious abnormalities in the posterior fossa were found on the initial computed tomography (CT) scans on the 12th day (Figure 1K-1I). About 2 weeks later, her MR images (Figure 1M-1T) showed abnormal signals in the posterior fossa with restricted diffusion (Figure 1R,1S) and significant enhancement of the lesion’s margin (Figure 1T), which supported the diagnosis of infratentorial subdural empyema. At the same time, her MRI examination revealed the presence of hydrocephalus (Figure 1M,1N), ventricular empyema (Figure 1M: DWI), and ventriculitis [Figure 1N: contrast-enhanced (CE)-MRI]. Meropenem was given intravenously to control infection. External ventricular drainage (EVD) was recommended to alleviate the severe intracranial complications. However, her parents declined and requested her discharge after about 2 weeks of anti-infection treatment, despite her infection indicators remaining abnormal. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patients’ guardians for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Subdural empyema in the posterior fossa is a severe condition in neurology with a high mortality and poor prognosis. Previous studies have indicated that more than half of the cases are secondary to ear infections, including suppurative otitis media and mastoiditis (3-5). However, subdural empyema in the posterior fossa caused by bacterial meningitis in children is extremely rare, let alone in newborns. Neuroimaging examinations, especially MRI, are indispensable for assisting its prompt and accurate diagnosis. Only one case has been reported by Tanaka et al., which was a 5-day-old girl with bilateral encapsulated low-density and edge-enhanced lesions on follow-up CT scans. However, this patient did not undergo the MR examination (6). Studies have indicated that the diagnostic value of CT is limited, which may lead to the delayed diagnosis of infratentorial subdural empyema (6,7). Our study fills this gap by describing the MRI findings of subdural empyema in the posterior fossa secondary to neonatal bacterial meningitis. At the same time, the recognition of MRI manifestations about infratentorial subdural empyema is helpful to distinguish it from other conditions in the same region such as venous sinus thrombosis and subdural hemorrhage, which also occur commonly in the neonatal period with different treatment.
In our study, both the subdural empyema cases in the posterior fossa secondary to neonatal bacterial meningitis were located on the convex surface of the cerebellum, which is the most common location for infratentorial subdural empyema (5). To be more precise, the pus accumulated in the subdural space, which is in front of the main venous sinus of the posterior fossa. The venous sinus could be detected behind the abnormal signal of subdural empyema with a low signal on T1WI, T2WI, and FLAIR (as the arrows indicated in the Figure 1) in our cases, which was further supported by MRV examinations (Figure 1G,1H) with the corresponding period. Therefore, observing the relationship between the lesion and the venous sinus on multiple planes and sequences can help to determine the location of the lesion as well as to distinguish it from cerebral venous sinus thrombosis.
In general, subdural empyema in the posterior fossa shows a slightly low signal on T1WI. However, it should be noted that both of our cases presented with discontinuous high signals at the edge of the lesion on T1WI (Figure 1A,1O,1P), which makes it necessary to differentiate them from the more common condition of subdural hemorrhage in the neonatal period (8). The high signal of the lesion’s edge on T1WI corresponds to subacute early hemorrhage with the low signal on T2WI, which is opposite to the actual situation (Figure 1B,1Q). In addition, the high signal of subdural hemorrhage on T1WI is usually continuous and obvious. The discontinuous high signal on the T1WI, as well as the low signal on T2WI at the edge of the cases, can be interpreted as a small amount of blood oozing (Figure 1A,1B,1O-1Q).
We have emphasized that restricted diffusion and prominent enhancement of the margin are typical features of subdural empyema. Restricted diffusion (Figure 1D,1E,1R,1S) is caused by the accumulation of purulent material, which contains a high concentration of cells and protein complexes that impede the free movement of water molecules (9). The enhanced border (Figure 1F,1T, as well as its blood oozing is attributed to the inflammatory response to the infection and the breakdown of the blood-brain barrier (BBB), allowing for permeability to contrast agents and blood components (10).
Posterior fossa subdural empyema can be caused by various pathogens, and the CSF culture of our two cases was positive for Escherichia coli (4). Although in the previous report by Tanaka et al. no pathogenic bacteria were isolated from the CSF or blood cultures, the causative organism might have also been Escherichia coli, which was suggested by the positive result of her mother’s vaginal mucus culture (6).
In general, surgical evacuation of the empyema combined with antibiotic therapy is the main treatment of subdural empyema, whereas surgical intervention can be avoided if treatment with antibiotics is effective (4,5). Although posterior fossa subdural empyema carries a high risk of causing brain herniation, the open fontanelle acts as a cushion against intracranial hypertension in the neonatal period and simple subdural empyema can be completely absorbed after antibiotic therapy, just as it was in the first case in our study. However, the management of these patients will become complicated when they are combined with hydrocephalus. Conservative treatment of hydrocephalus is acceptable if the pus is gradually absorbed after antibiotic therapy, or it is evacuated after surgery. EVD can be applied to deal with hydrocephalus in the acute situation (4). Rational management of hydrocephalus will contribute to improve the prognosis of patients.
Conclusions
Posterior fossa subdural empyema secondary to neonatal bacterial meningitis lacks specific clinical manifestations. Neuroimaging examinations, especially MRI, are indispensable for assisting its prompt and accurate diagnosis. Determining the lesion’s accurate location, signal characteristics, and enhancement pattern are of vital importance in clinical practice. Posterior fossa subdural empyema is usually located bilaterally in the convex surface of the cerebellum, which is confined to the front of the venous sinus. Multiplanar observations combined with MRV can help to distinguish it from venous sinus thrombosis. Restricted diffusion and enhanced margin are its representative characteristics. Blood oozing from the lesion’s edge with the discontinuous hyperintense can be observed on T1WI.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1113/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patients’ guardians for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Muzumdar D, Biyani N, Deopujari C. Subdural empyema in children. Childs Nerv Syst 2018;34:1881-7. [Crossref] [PubMed]
- Liu ZH, Chen NY, Tu PH, Lee ST, Wu CT. The treatment and outcome of postmeningitic subdural empyema in infants. J Neurosurg Pediatr 2010;6:38-42. [Crossref] [PubMed]
- van de Beek D, Campeau NG, Wijdicks EF. The clinical challenge of recognizing infratentorial empyema. Neurology 2007;69:477-81. [Crossref] [PubMed]
- Madhugiri VS, Sastri BV, Bhagavatula ID, Sampath S, Chandramouli BA, Pandey P. Posterior fossa subdural empyema in children--management and outcome. Childs Nerv Syst 2011;27:137-44. [Crossref] [PubMed]
- Venkatesh MS, Pandey P, Devi BI, Khanapure K, Satish S, Sampath S, Chandramouli BA, Sastry KV. Pediatric infratentorial subdural empyema: analysis of 14 cases. J Neurosurg 2006;105:370-7. [Crossref] [PubMed]
- Tanaka Y, Kobayashi S, Yokoh A, Takei M, Ohtake T. Posterior fossa subdural empyema in the term neonate. Childs Nerv Syst 1989;5:364-6. [Crossref] [PubMed]
- Rebchuk AD, Chang SJ, Griesdale DEG, Honey CR. Non-contrast-enhancing subdural empyema: illustrative case. J Neurosurg Case Lessons 2022;4:CASE22269. [Crossref] [PubMed]
- Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, Gilmore JH. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology 2007;242:535-41. [Crossref] [PubMed]
- Wong AM, Zimmerman RA, Simon EM, Pollock AN, Bilaniuk LT. Diffusion-weighted MR imaging of subdural empyemas in children. AJNR Am J Neuroradiol 2004;25:1016-21.
- Tsuchiya K, Makita K, Furui S, Kusano S, Inoue Y. Contrast-enhanced magnetic resonance imaging of sub- and epidural empyemas. Neuroradiology 1992;34:494-6. [Crossref] [PubMed]


