
State of the art in the diagnostic evaluation of osteomyelitis: exploring the role of advanced MRI sequences—a narrative review
Introduction
Osteomyelitis is an acute or chronic inflammatory process involving the bone and its structures secondary to infection and can arise as a result of the spread of pathogens through the bloodstream from a remote location, through direct introduction following an injury, or by extending from a nearby soft tissue infection. It can be caused by viruses, bacteria, fungi, and parasites but the majority are caused by bacteria. The most common causative agent is Staphylococcus aureus, responsible for 80–90% of pyogenic osteomyelitis (1). In osteomyelitis, magnetic resonance imaging (MRI) remains the imaging modality of choice after initial evaluation through plain radiography (2,3). Conventional MRI sequences have long been the cornerstone in the diagnosis of osteomyelitis, offering notable advantages such as excellent soft tissue contrast and multiplanar imaging capabilities (4). It has high sensitivity and specificity in the diagnosis of osteomyelitis as described in the recent works by Weaver et al. and Llewellyn et al. showing that in adults, MRI had a sensitivity of 95.6% and a specificity of 80.7% in the diagnosis of osteomyelitis (1,5). T1-weighted and T2-weighted sequences, along with gadolinium-enhanced T1-weighted imaging, have been instrumental in visualizing bone marrow edema, soft tissue abscesses, and periosteal reactions, facilitating the accurate assessment of osteomyelitis (4).
However, these conventional sequences come with limitations, such as reduced sensitivity in the early stages of the disease and the inability to differentiate between infectious and non-infectious causes of bone marrow edema (4). Indeed, bone marrow signal abnormalities on MRI are known to be non-specific findings (differential diagnosis includes contusion, fracture, postsurgical change, arthritis, neoplasm, Charcot arthropathy, etc.) and can overestimate the extent and duration of infection on fluid-sensitive images due to the coexistence of reactive marrow edema with true marrow infection (2,3).
In contrast, innovative approaches like diffusion-weighted imaging (DWI) and dynamic contrast-enhanced MRI (DCE-MRI) have emerged as potential game-changers in osteomyelitis diagnosis. DWI provides insights into tissue cellularity and can detect subtle changes even before structural alterations become apparent. DCE-MRI, on the other hand, aids in assessing perfusion dynamics, offering valuable information about the inflammatory process (6). In this review article we investigate the role of such advanced MR sequences such as DCE-MRI, DWI, magnetic resonance spectroscopy (MRS), and Dixon in the evaluation of osteomyelitis. We present this article in accordance with the Narrative Review reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1138/rc).
Methods
The systematic literature search conducted on March 26, 2023, aimed to identify relevant studies pertaining to advanced MRI techniques in the context of osteomyelitis. The primary database searched was PubMed, utilizing a comprehensive set of search terms including “(advanced MRI techniques) AND (osteomyelitis)”. The search timeframe spanned from 1988 to 2023 to ensure a comprehensive coverage of relevant literature. Inclusion criteria were established to encompass a broad spectrum of evidence, comprising all types of studies such as reviews, letters, and case reports. Additionally, reports written in either English or French were eligible for review, and human studies were considered. Conversely, exclusion criteria were defined to refine the focus, excluding animal studies and articles not written in English or French. The selection process involved both reviewers independently screening the identified records. After the independent selection, the reviewers compared their choices, and any disparities were resolved through discussion, emphasizing a consensus-based approach. This method was employed to enhance the reliability and objectivity of the study selection process. For a summary of the review process see Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 26 March 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | (advanced MRI techniques) AND (osteomyelitis) |
| Timeframe | 1988–2023 |
| Inclusion and exclusion criteria | Inclusion criteria: (I) all types of studies including reviews, letters, and case reports; (II) reports written in English or French; and (III) human studies were eligible for review. Exclusion criteria: (I) animal studies and (II) articles not written in either English or French were excluded |
| Selection process | Both reviewers selected the records independently and compared afterwards. Any disagreements were resolved through discussion |
DWI
DWI is a molecular method for evaluating bone marrow edema that makes use of the random (Brownian) movement of free water molecules inside a tissue voxel to produce an indirect estimate of cellularity and cell membrane integrity (7). The accuracy of DWI also increases as the level of diffusion weighting (b value or diffusion moment) increases (7). The measurement of diffusion in the form of apparent diffusion coefficient (ADC) involves data acquisition with two distinct b values. Unlike conventional DWI, which inherently carries T2-weighting, ADC values are devoid of this property, and they are presented in the form of a parametric map, commonly referred to as an ADC map (7). Each voxel within the ADC map represents a distinct numerical diffusion value, expressed in units of mm2/s. By selecting a region of interest (ROI) on the ADC map, we can ascertain the average ADC values (3).
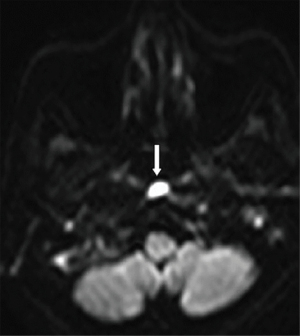
DWI could have a valuable role in osteomyelitis by assisting in the characterization of associated fluid collections. In cases where purulent material is present within an abscess, the free diffusion of water molecules will be restricted. This restriction will be reflected in high diffusion signals on fractional anisotropy/trace images and low signal intensity on ADC maps, resulting in an improved image with excellent background suppression, as illustrated in Figure 1 (7-9). This use case becomes especially important for individuals who are unable to be administered gadolinium-based contrast agents because of compromised renal function or an allergy to contrast agents (9-13).
Another valuable application of DWI is its potential to differentiate bone infarct from osteomyelitis in sickle cell patients, which is considered one of the most challenging issues both radiologically and clinically when evaluating acute bone pain in this patient population (14,15). In these scenarios, achieving an early diagnosis holds significant therapeutic and prognostic implications, as it permits the implementation of specific treatments at an initial stage, thereby circumventing the unnecessary use of intravenous broad-spectrum antibiotics, minimizing the requirement for multiple diagnostic assessments, reducing morbidity, shortening hospital stays, and resulting in substantial cost savings (14,15). While both bone infarct and osteomyelitis can manifest restricted diffusion, characterized by a bright DWI signal and a low ADC value, Tuna et al. proposed that during the initial stages of a vaso-occlusive crisis (within 24 hours), there may be restricted diffusion associated with a dark DWI signal and diminished T2 signal. This distinction could prove beneficial in discerning between a bone infarct and an infectious process (14). Additional supporting factors for diagnosing bone infarction instead of infection include the lack of edema on T2-weighted images, the lesion’s geographical pattern, poor enhancement of the infarcted bone, and the absence of permeative or erosive changes. Figure 2 illustrates this differentiation (14). It’s crucial to recognize that the presence of sickling and sequestration can result in an exceptionally low signal on T2-weighted images, primarily due to the paramagnetic influence of intracellular deoxyhemoglobin (14). Given that diffusion-weighted images inherently incorporate T2 weighting, the tissue’s T2 properties can impact their appearance, irrespective of tissue diffusivity. This can give rise to T2 shine-through and T2 blackout effects (14). The phenomenon of DWI T2 blackout occurs when lesions with extremely low T2 values diminish the signal intensity in the diffusion-weighted image, potentially concealing or eliminating its sensitivity to diffusion. In severe instances, this may have implications for the accuracy of the ADC map calculations, rendering it unreliable (14,16).
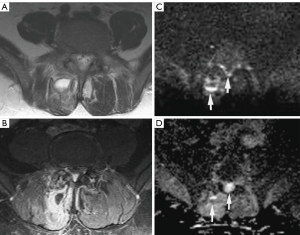
Chaturvedi (8) highlights that DWI is also valuable in distinguishing between bone malignancy and aggressive osteomyelitis. Similarly, a study by Leclair et al. showed a substantial elevation of the ADC values in inflammatory bone lesions due to chronic recurrent nonbacterial osteomyelitis (17). These studies highlighted DWI as a promising technique that may help to distinguish between malignant lesions and benign inflammatory processes (8). Furthermore, in cases of bacterial Skull Base Osteomyelitis (SBO), DWI aids in its differential diagnosis with lymphoma or nasopharynx carcinoma (18). Ozgen et al. demonstrated that using an ADC value cutoff equal to or higher than 1.08×10−3 mm2/s can effectively rule out lymphoma and nasopharynx carcinoma with an accuracy of 96% (19). Similarly, this ADC cutoff can be utilized to distinguish between patients with SBO and patients with neoplastic lesions of the skull base (specifically lymphoma, nasopharynx carcinoma, and metastasis), achieving an accuracy of 86%, with a sensitivity and specificity of 89% and 85%, respectively (19). In bacterial SBO, ADC values tend to be high, whereas in malignant diseases, ADC values tend to be relatively reduced due to factors such as reduced extracellular matrix, enlargement of nuclei, and hypercellularity (18). However, the presence of restricted diffusion, increased signal on DWI, and low ADC values should raise suspicion of associated abscess formation in SBO (similar to intraosseous abscesses) (18,20). One limitation of DWI is its inability to differentiate between abscess formation in SBO and malignant neoplasms (21). Nevertheless, on postcontrast imaging, focal abscesses may exhibit a peripheral rim of enhancement, whereas neoplasms generally show homogenous or heterogenous enhancement within the diffusion-restricting tissue. Refer to Figure 3 for an illustration (21). A significant hurdle encountered with diffusion-weighted images in the skull base region pertains to susceptibility artifacts stemming from the non-uniform tissues in this region (18). Nevertheless, the utilization of non-epi DWI imaging can offer a solution to mitigate these artifacts (18).
DWI is also utilized for distinguishing between diabetic foot osteomyelitis (DFO) and Charcot neuro-osteoarthropathy (CN). In a study conducted by Diez et al., the researchers aimed to compare the diagnostic accuracy of functional MRI with that of 18F-FDG PET/CT in differentiating between DFO and CN (22). Their findings indicated that analyzing the high b-value signal pathological-to-normal bone ratio on DWI (DWIr) and measuring the volume transfer constant (Ktrans) and internal area under the gadolinium curve at 60 s (iAUC60) allowed for a reliable differentiation of DFO and CN, especially for large regions of interest (ROIs) (22). While 18F-FDG PET/CT was identified as the most accurate diagnostic modality for differentiating these conditions, the novel combined PET/MRI, which integrates morphological, functional, and metabolic evaluations, shows great promise as a pivotal tool in DFO imaging (22-26). This integrated approach may provide valuable insights and improved accuracy in distinguishing between DFO and CN, benefiting patients and clinicians in managing these complex conditions more effectively.
In a comparative study conducted by Habre et al. (27), the diagnostic performance of standard contrast-enhanced MRI was compared to an unenhanced DWI protocol (11). The results revealed that emergency unenhanced DWI alone is adequate for establishing the diagnosis of pediatric acute osteoarticular infections, eliminating the need for gadolinium contrast. Specifically, for septic arthritis and osteomyelitis in long bone metaphyses/diaphyses or metaphyseal equivalents, DWI did not provide additional diagnostic value since all cases were effectively identified using standard unenhanced MRI sequences (11). On the other hand, when it comes to the diagnosis of bone and soft tissue abscesses, DWI significantly improved sensitivity to 100%. The imaging protocol, which included short tau inversion recovery (STIR), water-only T2 Dixon, T1, and DWI, proved sufficient for enabling prompt medical and surgical management of these conditions (27). However, it’s important to note that static contrast-enhanced MRI remains crucial for evaluating the femoral head chondroepiphysis in children below the age of 30 months, especially considering their increased propensity for this site (27,28). In this particular age group, DWI has limited sensitivity, which is partly explained by the low epiphyseal fat content in younger individuals, leading to higher enhancement that may affect the effectiveness of DWI in this specific area (27). Overall, the study suggests that an unenhanced DWI protocol can be highly valuable in the diagnosis of pediatric acute osteoarticular infections, offering a reliable alternative to standard contrast-enhanced MRI, while still recognizing the importance of using contrast-enhanced MRI for specific scenarios, such as evaluating the femoral head chondroepiphysis in young children.
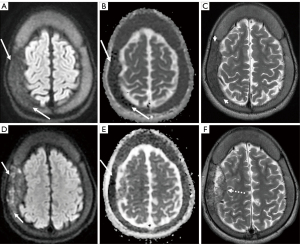
DWI also proves advantageous in distinguishing acute discitis-osteomyelitis from Modic I degenerative alterations, as these two conditions can exhibit similar MRI appearances (1). Modic type 1 degenerative signal changes can occasionally resemble infections, prompting invasive diagnostic procedures and incurring additional costs (1). However, in a retrospective study by Patel et al., the accuracy and utility of a novel DWI “claw sign” were analyzed to distinguish vertebral diskitis/osteomyelitis from symptomatic type 1 degeneration (1,29). The study revealed that the presence of a “claw sign” is highly suggestive of degenerative disease, with a very high likelihood (97–100%) of being associated with degeneration rather than infection. Conversely, the absence of the “claw sign”, which refers to a pattern of diffusely increased diffusion signal, strongly suggests diskitis/osteomyelitis in individuals displaying type 1 signal changes in the vertebral bodies exhibiting a notably high accuracy, ranging from 93% to 100%, in effectively discerning infection from disk degeneration associated with Modic type 1 endplate alterations (1,29). Figure 4 illustrates this finding. This differentiation is valuable in clinical practice as it helps avoid unnecessary invasive procedures and reduces healthcare costs by guiding appropriate management based on the underlying condition, whether it is degenerative changes or infection.
Diagnosing spinal infections can be challenging as MRI results frequently trail behind the onset of clinical symptoms, and may also take time to normalize during the healing phase. This uncertainty can make it difficult to initiate treatment and determine when treatment has been adequate to be stopped (30). In a study conducted by Dumont et al., they proposed standards for ADC values that are representative of spinal infection (30). Their research revealed that in individuals suspected of having a spine infection, the ADC values obtained from DWI were notably lower in those with positive microbiological sampling (indicating the presence of infection) as opposed to individuals with negative microbiological sampling (indicating the absence of infection) (30). In accordance with their investigation, an ADC value falling below 1,250×10−6 mm2/s could be a cause for concern when evaluating the possibility of spine infection. Furthermore, their observations indicated that ADC values may return to normal levels during successful treatment of spinal infections, implying that DWI can serve as a valuable tool for monitoring the response to antibiotic therapy (30,31). This information can complement conventional MRI, especially when the clinical picture is equivocal, helping in more accurate and timely decision-making regarding the diagnosis and management of spinal infections (30). By utilizing DWI as an adjunct to conventional MRI, healthcare professionals can potentially improve the accuracy of diagnosing spinal infections and assess treatment response more effectively, ultimately leading to better patient outcomes.
Non contrast DWI sequences have been found to improve specificity of conventional MR sequences in diagnosing pedal osteomyelitis, as conventional MR sequences alone can lead to false positive results (6). By using an ADC value cut-off of 1.57×10−3 mm2/s, the sensitivity for diagnosing pedal osteomyelitis was 88.2%±10.3%, while the specificity was 80.0%±19.4% (6). This increased specificity is especially beneficial for patients with pedal osteomyelitis, as they often have impaired renal function that may prevent the use of contrast agents in conventional MR sequences. Non-contrast diffusion-weighted sequences provide valuable diagnostic information without the need for contrast, offering a safer and more reliable imaging option in this specific patient population (6). By incorporating non-contrast diffusion-weighted sequences in addition to conventional MR sequences, healthcare professionals can more accurately distinguish pedal osteomyelitis from other conditions, leading to improved diagnoses and better patient care.
DCE-MRI
The increasing body of research in the realm of MRI physics has brought forth the development of novel sequences that incorporate functional imaging to enhance the specificity of this imaging modality. DCE-MRI is an example of such an approach, which evaluates the progression of contrast agent uptake in a lesion over a period of time (6,32).
IV gadolinium contrast is recommended when there is suspicion of epiphyseal infection because unenhanced images may not show any abnormalities, potentially leading to a false-negative diagnosis. By using post-contrast fat-saturated T1 sequences, any sinus tract or abscess can be better visualized and characterized, enhancing the detection and evaluation of the infection. Moreover, IV gadolinium contrast is essential for distinguishing between an abscess and a phlegmon. The contrast agent aids in highlighting areas with increased vascularity, allowing for a clearer differentiation between these two conditions. This differentiation is crucial for appropriate treatment planning and management decisions.
Raj et al. conducted a study focused on examining the contrast uptake patterns in two groups of patients suffering from diabetic foot ulcers and suspected DFO (6). They utilized semi-quantitative parameters from the DCE-MRI sequence, which had not been previously assessed for diagnosing DFO. Additionally, they compared the differences in ADC values between these patient groups. The study findings demonstrated that the sensitivity of MRI for diagnosing DFO can be enhanced by incorporating DCE-MRI alongside conventional MRI sequences (6). The DCE parameters, including tissue signal intensity on unenhanced T1 images (SI0), maximum absolute contrast enhancement (SImax), wash-in rate (WIR), and the type of contrast enhancement curve, were found to be valuable in detecting DFO. WIR and the presence of a type II enhancement kinetic curve (observed in 94.1% of positive cases) were particularly advantageous for diagnosing osteomyelitis (6). This approach can enhance the overall sensitivity and specificity of MRI in distinguishing DFO from acute Charcot arthropathy, thereby addressing diagnostic challenges (6). WIR provided insights into the rate of contrast uptake within a lesion, and it was significantly higher in cases of biopsy-confirmed DFO compared to biopsy-negative cases. This difference was attributed to increased vascularity, hyperemia, and vasodilation resulting from the presence of various inflammatory cytokines that promote transudation across capillaries during infection (6). In contrast, the lower levels of inflammation and hyperemia in acute Charcot led to a distinct contrast uptake pattern. The higher tissue signal intensity on unenhanced T1 images (SI0) and maximum absolute contrast enhancement (SImax) observed in affected bones were likely due to the presence of more inflammatory cells and heightened hyperemia resulting from the infection (6).
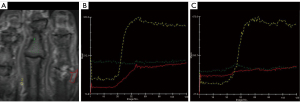
DCE-MRI offers the advantage of providing quantitative assessments of perfusion and permeability, making it an effective tool for investigating microcirculation in living tissues that conventional MRI cannot evaluate. Notably, a previous study conducted by Jans et al. illustrated that in cases of osteomyelitis, the time-intensity curve (TIC) displayed an initial rapid rise followed by a progressively slower increase, potentially indicating the presence of an inflammatory process. In contrast, in areas affected by oedema in neuropathic arthropathy, a lower enhancement rate was observed with a slightly progressive increase in the TIC slope relative to the baseline, as depicted in Figure 5 (33). Differentiating between regions presenting simultaneous osteomyelitis and acute neuropathic arthropathy poses an extremely challenging task for both clinicians and radiologists (34). In a study by Liao et al. aimed at evaluating the possibility of using DCE-MRI to differentiate between these two conditions, it was shown that the DCE-MRI parameters of the osteomyelitis group included greater transfer constant Ktrans (related to wash-in), rate contrast Kep (related to washout), and extracellular extravascular space volume fraction Ve values than those of the acute neuropathic arthropathy group (34). This distinction can be elucidated by considering the distinct pathological characteristics of these lesions. Acute neuropathic arthropathy is primarily associated with bone marrow edema, characterized by relatively lower cellularity. In contrast, osteomyelitis involves the presence of white blood cells, deceased microorganisms, inflammatory cells, and, consequently, higher cellularity, leading to greater permeability (34). Furthermore, Pearson correlation analysis demonstrated a significant correlation between these DCE quantitative permeability parameters and the CRP and ESR levels in osteomyelitis patients (34). One possible explanation for this phenomenon may be the presence of microvascular irregularities within the inflammatory regions (35). In fact, Ertugrul et al. emphasized the significance of erythrocyte sedimentation rate (ESR) as a key inflammatory factor in diagnosing osteomyelitis (36). Furthermore, Van Asten et al. highlighted the importance of ESR and C-reactive protein (CRP) levels as crucial inflammatory markers for monitoring the effectiveness of therapy in osteomyelitis treatment (37). Consequently, the observed correlations between DCE-MRI parameters and inflammatory markers suggest that DCE-MRI parameters could serve as valuable imaging biomarkers, offering insights into the inflammatory microenvironment of osteomyelitis (since ESR and CRP are non-specific and may be positive even in other concomitant inflammatory conditions) and might be predictive of patient prognosis (38). This is because, apart from being non-specific to osteomyelitis, CRP and ESR do not give enough information on soft tissue involvement in chronic osteomyelitis, and thus could be useful in certain situations, it could serve as an alternative to performing an 18F-FDG PET exam or a painful bone biopsy (39).
Dixon acquisition
The main types of MRI fat suppression techniques include chemical shift selective fat saturation pulse sequences (CHESS), STIR sequences, SPectral Attenuated Inversion Recovery (a hybrid technique combining features of both CHESS and STIR and based on chemical selective inversion and an adiabatic pulse), and chemical shift-based water/fat separation sequences like Dixon sequence techniques (40). These techniques exploit the chemical shift phenomenon, which allows the separation of water and fat signals based on their different resonant frequencies (7,41). In recent years, technical advancements have improved the optimization of the chemical shift phenomenon in Dixon acquisitions, enabling the generation of water-only signal images (by adding both in-phase and out-of-phase signal intensities) and fat-only signal images (by subtracting the in-phase signal intensity from that of the out-of-phase image) within a single acquisition using multiple echoes in the same sequence, with the assistance of mathematical postprocessing of data (42). Unlike other MR fat suppression techniques that suppress fat during image acquisition, the Dixon technique suppresses fat in post-processing (43). Additionally, the Dixon method is relatively less sensitive to susceptibility artifacts, resulting in better detection of bony vertebral pathologies (44,45). The application of Dixon sequences enhances image quality and increases the detection of both soft tissue and osseous abnormalities, such as intraosseous sequestrums, sinus tracts, fistulas, cortical destruction, and periostitis. This improved visualization facilitates the differentiation between diabetic foot and osteomyelitis, as illustrated in Figure 6 (10,46). By utilizing Dixon sequences, healthcare professionals can obtain clearer and more detailed images, leading to better diagnostic accuracy and more effective management of foot and bone conditions.
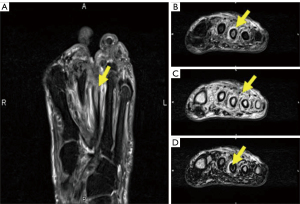
Dixon sequences could be used to distinguish between osteomyelitis from neuropathic arthropathy since neuropathic arthropathy shows a signal loss in the opposed-phase (OP) in the evaluation of bone marrow edema whereas there is no significant signal loss in osteomyelitis. This is because in OP, the signal is the difference between the signals from water and fat molecules (water signal-fat signal), OP images show a reduced signal from voxels that contain both fat and water. Thus, the increased edema in neuropathic arthropathy explains the signal loss on OP imaging compared to osteomyelitis which shows a severe increase in both water content and fat content (due to the high cellularity given predominantly by inflammatory cells) early in the course of the disease (10). In cases where only neuropathic arthropathy is present, genuine bone destruction occurs, resulting in the relative disappearance of bone contours on T1-weighted images. These contours do not reappear on fat-suppressed images. However, when both osteomyelitis and neuropathic arthropathy coexist, the bone contours also tend to disappear on T1-weighted images, but they have a tendency to re-emerge on fat-suppressed images, a phenomenon commonly referred to as the “ghost sign” (47). Dixon imaging is also a fundamental component of adequate evaluation of the diabetic foot.
Proton MR spectroscopy
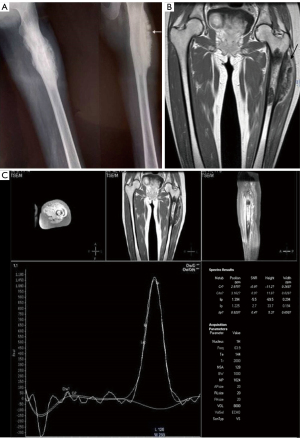
Proton MR spectroscopy is a well-established imaging modality that provides valuable chemical and metabolic information about lesions by generating a spectrum of signal intensities emitted from different tissue metabolites. One essential capability of MR spectroscopy is its ability to reliably detect various lipid compartments (25,26,48,49). Notably, it can assess the unsaturation levels among triglycerides, which have proven useful in quantitatively assessing bone marrow inflammatory lesions (50). Another valuable aspect of proton MR spectroscopy is its ability to detect choline, a marker for cell membrane turnover. This enables differentiation between malignant and benign lesions (51,52). Amar et al. demonstrated that the absence of a choline peak on 1H-MRS in focal bone lesions provides reliable assurance against malignancy (except for giant cell tumors), as illustrated in Figure 7 (53). However, using MR spectroscopy in bone lesions poses some challenges. The inherent heterogeneity of bone, with osseous trabeculae, fat, vessels, and muscle, along with magnetic susceptibility, makes adequate shimming (a process to optimize magnetic field homogeneity) a daunting task. This often leads to a high probability of obtaining inadequate spectra due to the abundance of lipids present in the musculoskeletal system (54). Additionally, high levels of creatine in muscle can mask smaller metabolites, further complicating the interpretation of MR spectra (54). As of now, functional MRI imaging techniques such as MRI perfusion or MRS have not been widely utilized in the evaluation of osteomyelitis. While some studies suggest that these techniques could aid in making the differential diagnosis, more research should be encouraged before any definitive conclusions can be drawn (25,26). Further investigations and studies are necessary to determine the potential role of these functional MRI techniques in improving the evaluation and diagnosis of osteomyelitis.
Feasibility and challenges
It is crucial to note that these innovative sequences are not yet part of routine MRI examinations for osteomyelitis, and their incorporation into clinical practice requires careful consideration. As for the technical aspects, the feasibility of performing these sequences on standard MRI apparatus varies. While some, such as DCE-MRI and DWI, can be executed on conventional scanners with appropriate parameter adjustments, others may necessitate specialized hardware configurations. In particular, performing MR spectroscopy and Dixon imaging in standard MRI machines is not possible without specialized hardware and software (55). Both of these techniques demand unique equipment and dedicated software for accurate results. For MR spectroscopy, specialized radiofrequency coils and pulse sequences are essential to target specific regions and measure chemical compounds. Post-processing software is required to analyze the acquired data and generate spectra. Dixon imaging, used for fat-water separation, relies on acquiring multiple images at different echo times and then applying complex mathematical algorithms during post-processing to create separate fat and water images. If an MRI machine lacks the necessary hardware and software for these techniques, upgrading the equipment or seeking access to a facility with specialized MRI capabilities should be considered. Thus, standard MRI software alone does not have the capabilities to perform MR spectroscopy or Dixon imaging (55). In addition, the increased scan times and complexity associated with some of these sequences (such as DCE-MRI) may raise concerns regarding increased costs for healthcare providers and patient tolerability, which demand further evaluation. Regarding the expertise required, the latter sequences cannot be performed by all radiologists and their application is limited to specialized radiology departments (56,57). Adequate training and familiarity with these novel techniques are pivotal to ensure accurate and reliable results. While innovative MRI sequences hold promise in the diagnosis of osteomyelitis, their integration into standard practice necessitates addressing technical, logistical, and expertise-related challenges.
Conclusions and future outlook
Standard T1 and T2-weighted MRI is typically based on morphological sequences that provide purely structural information. It is clear that the above-discussed technical improvements (summarized in Table 2) have allowed the incorporation of functional quantitative information into structural information. As an illustration, a prior investigation conducted by Baba et al. demonstrated that quantitative parameters from DCE-MRI and normalized ADC values could be valuable in distinguishing between skull base osteomyelitis and nasopharyngeal cancer. Interestingly, when used in combination, these DCE-MRI parameters and normalized ADC values yielded better diagnostic performance compared to using each measure independently (59). Thus, a hypothesis worth being investigated in future is whether two or more advanced techniques in the setting of MR imaging could be combined to improve the diagnostic efficiency of osteomyelitis. Furthermore, one of the latest studies showed that Chemical shift imaging and related Dixon sequence were reliable tools for evaluating diabetic foot. They could distinguish between mimickers of bland edema of osteomyelitis and infectious edema-like changes of osteomyelitis-like changes with high sensitivity and specificity, particularly when using quantitative analysis of their signal abnormality (60).
Table 2
| Application | Imaging findings and indications | Supplementary data | References |
|---|---|---|---|
| To characterize osteomyelitis associated purulent abscess | ↑ DWI signal & ↓ ADC map signal | Patients with contraindication to gadolinium-based contrast agents | (3,4,7,13-16,25,26,58) |
| To differentiate bone infarct from osteomyelitis | Osteomyelitis: ↑ DWI signal & ↓ ADC map signal, ↓ T2 signal; in the early phases of vaso-occlusive crisis (within 24 h) of bone infarct: ↓ DWI signal & ↓ ADC map signal, ↓ post-contrast T2 signal | Sickle cell patients | (8) |
| To distinguish between abscess formation in SBO and malignant neoplasms of the skull base | Abscess formation in SBO: ↑ DWI signal & ↓ ADC map signal; post-contrast peripheral rim enhancement; malignant skull base neoplasms: ↑ DWI signal & ↓ ADC map signal; post-contrast enhancement within the diffusion-restricting tissue | (7,9,44,45) | |
| To differentiate diabetic foot osteomyelitis from Charcot neuro-osteoarthropathy | DCE-MRI parameters such as DWIr, Ktrans, iAUC60, WIR and type II enhancement (time-intensity) kinetic curve; utilize 18F-fluorodeoxyglucose positron emission tomography/computed tomography whenever magnetic resonance imaging is unavailable or inconclusive | (10,18) | |
| To differentiate acute discitis-osteomyelitis from Modic I degenerative changes | Modic type I degenerative changes: ↑ DWI signal + DWI claw sign; acute discitis/osteomyelitis: ↑ DWI signal + absence DWI claw sign | (1,9) | |
| In treatment planning for spinal infections | ADC value <1,250×10−6 mm2/s is indicative of ongoing spine infection; ADC values would normalize during effective treatment of spine infection | In patients with spinal infections whenever conventional MRI clinical picture is inconclusive | (17) |
| For more accurate diagnosis of pedal osteomyelitis | ADC value cut-off of 1.57×10−3 mm2/s | In patients with deranged renal parameters | (18) |
| For suspected epiphyseal infection +/− sinus tracts or abscesses with normal unenhanced images | Post-contrast FS-T1 sequences will allow further characterization | (6) | |
| To distinguish osteomyelitis from neuropathic arthropathy | In osteomyelitis: an initially fast-rising slope, followed by a progressively slow-rising phase observed on the time-intensity curve; no significant signal loss in Dixon sequence opposed-phase; in neuropathic arthropathy: a lower enhancement rate with a slightly progressive increase in the TIC slope relative to the baseline; significant signal loss in Dixon sequence opposed-phase | In patients whom an 18F-FDG PET exam or a painful bone biopsy is not preferred | (4,32) |
| To differentiate malignant from benign lesions | Absence of choline peak on 1H-magnetic resonance spectroscopy in focal bone lesions is a reliable assurance against malignancy (with exception of giant cell tumors) | In patients with suspected bone malignancy | (38-40,42) |
DWI, diffusion weighted imaging; ADC, apparent diffusion coefficient; SBO, skull base osteomyelitis; DCE, dynamic contrast-enhanced; MRI, magnetic resonance imaging; DWIr, high b-value signal pathological-to-normal bone ratio on DWI; Ktrans, the volume transfer constant; iAUC60, internal area under the gadolinium curve at 60 s; WIR, wash-in rate; FS-T1, T1 fat-saturated; TIC, time intensity curve.
Despite these recent developments, the role of these advanced techniques in the setting of osteomyelitis has not been fully defined and thus, further research is necessary to fully define their utility in this setting.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1138/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1138/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weaver JS, Omar IM, Mar WA, Klauser AS, Winegar BA, Mlady GW, McCurdy WE, Taljanovic MS. Magnetic resonance imaging of musculoskeletal infections. Pol J Radiol 2022;87:e141-62. [Crossref] [PubMed]
- Theodorou SJ, Theodorou DJ, Resnick D. Imaging findings of complications affecting the upper extremity in intravenous drug users: featured cases. Emerg Radiol 2008;15:227-39. [Crossref] [PubMed]
- Kruk KA, Dietrich TJ, Wildermuth S, Leschka S, Toepfer A, Waelti S, Kim CO, Güsewell S, Fischer T. Diffusion-Weighted Imaging Distinguishes Between Osteomyelitis, Bone Marrow Edema, and Healthy Bone on Forefoot Magnetic Resonance Imaging. J Magn Reson Imaging 2022;56:1571-9. [Crossref] [PubMed]
- Lee YJ, Sadigh S, Mankad K, Kapse N, Rajeswaran G. The imaging of osteomyelitis. Quant Imaging Med Surg 2016;6:184-98. [Crossref] [PubMed]
- Llewellyn A, Kraft J, Holton C, Harden M, Simmonds M. Imaging for detection of osteomyelitis in people with diabetic foot ulcers: A systematic review and meta-analysis. Eur J Radiol 2020;131:109215. [Crossref] [PubMed]
- Raj S, Prakash M, Rastogi A, Sinha A, Sandhu MS. The role of diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging for the diagnosis of diabetic foot osteomyelitis: a preliminary report. Pol J Radiol 2022;87:e274-80. [Crossref] [PubMed]
- Rubitschung K, Sherwood A, Crisologo AP, Bhavan K, Haley RW, Wukich DK, Castellino L, Hwang H, La Fontaine J, Chhabra A, Lavery L, Öz OK. Pathophysiology and Molecular Imaging of Diabetic Foot Infections. Int J Mol Sci 2021;22:11552. [Crossref] [PubMed]
- Chaturvedi A. Pediatric skeletal diffusion-weighted magnetic resonance imaging, part 2: current and emerging applications. Pediatr Radiol 2021;51:1575-88. [Crossref] [PubMed]
- Moritani T, Kim J, Capizzano AA, Kirby P, Kademian J, Sato Y. Pyogenic and non-pyogenic spinal infections: emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. Br J Radiol 2014;87:20140011. [Crossref] [PubMed]
- Martín Noguerol T, Luna Alcalá A, Beltrán LS, Gómez Cabrera M, Broncano Cabrero J, Vilanova JC, Advanced MR. Imaging Techniques for Differentiation of Neuropathic Arthropathy and Osteomyelitis in the Diabetic Foot. Radiographics 2017;37:1161-80. [Crossref] [PubMed]
- Altmayer S, Verma N, Dicks EA, Oliveira A. Imaging musculoskeletal soft tissue infections. Semin Ultrasound CT MR 2020;41:85-98. [Crossref] [PubMed]
- Winegar BA, Kay MD, Taljanovic M. Magnetic resonance imaging of the spine. Pol J Radiol 2020;85:e550-74. [Crossref] [PubMed]
- Harish S, Chiavaras MM, Kotnis N, Rebello R. MR imaging of skeletal soft tissue infection: utility of diffusion-weighted imaging in detecting abscess formation. Skeletal Radiol 2011;40:285-94. [Crossref] [PubMed]
- Tuna IS, Tarhan B, Escobar M, Albayram MS. T2-blackout effect on DWI as a sign of early bone infarct and sequestration in a patient with sickle cell disease. Clin Imaging 2019;54:15-20. [Crossref] [PubMed]
- Mourad C, Cosentino A, Nicod Lalonde M, Omoumi P. Advances in Bone Marrow Imaging: Strengths and Limitations from a Clinical Perspective. Semin Musculoskelet Radiol 2023;27:3-21. [Crossref] [PubMed]
- Huang TH, Lai MC, Chen YS, Huang CW. Brain Imaging in Epilepsy-Focus on Diffusion-Weighted Imaging. Diagnostics (Basel) 2022;12:2602. [Crossref] [PubMed]
- Leclair N, Thörmer G, Sorge I, Ritter L, Schuster V, Hirsch FW. Whole-Body Diffusion-Weighted Imaging in Chronic Recurrent Multifocal Osteomyelitis in Children. PLoS One 2016;11:e0147523. [Crossref] [PubMed]
- van Kroonenburgh AMJL, van der Meer WL, Bothof RJP, van Tilburg M, van Tongeren J, Postma AA. Advanced Imaging Techniques in Skull Base Osteomyelitis Due to Malignant Otitis Externa. Curr Radiol Rep 2018;6:3. [Crossref] [PubMed]
- Ozgen B, Oguz KK, Cila A. Diffusion MR imaging features of skull base osteomyelitis compared with skull base malignancy. AJNR Am J Neuroradiol 2011;32:179-84. [Crossref] [PubMed]
- Kumar Y, Khaleel M, Boothe E, Awdeh H, Wadhwa V, Chhabra A. Role of Diffusion Weighted Imaging in Musculoskeletal Infections: Current Perspectives. Eur Radiol 2017;27:414-23. [Crossref] [PubMed]
- Jain N, Jasper A, Vanjare HA, Mannam P, Mani SE. The role of imaging in skull base osteomyelitis - Reviewed. Clin Imaging 2020;67:62-7. [Crossref] [PubMed]
- Diez AIG, Fuster D, Morata L, Torres F, Garcia R, Poggio D, Sotes S, Del Amo M, Isern-Kebschull J, Pomes J, Soriano A, Brugnara L, Tomas X. Comparison of the diagnostic accuracy of diffusion-weighted and dynamic contrast-enhanced MRI with (18)F-FDG PET/CT to differentiate osteomyelitis from Charcot neuro-osteoarthropathy in diabetic foot. Eur J Radiol 2020;132:109299. [Crossref] [PubMed]
- Arachchige ASPM. 7-Tesla PET/MRI: A promising tool for multimodal brain imaging? AIMS Neurosci 2022;9:516-8. [Crossref] [PubMed]
- Perera Molligoda Arachchige AS. Neuroimaging with PET/MR: moving beyond 3 T in preclinical systems, when for clinical practice? Clin Trans Imaging 2023;11:315-9.
- Perera Molligoda Arachchige AS. Neuroimaging with PET/MR: moving beyond 3 T in preclinical systems, when for clinical practice? Clin Transl Imaging 2023;11:315-9.
- Arachchige ASPM. Neuroimaging with SPECT-MRI: a myth or reality? AIMS Neurosci 2023;10:52-5. [Crossref] [PubMed]
- Habre C, Botti P, Laurent M, Ceroni D, Toso S, Hanquinet S. Benefits of diffusion-weighted imaging in pediatric acute osteoarticular infections. Pediatr Radiol 2022;52:1086-94. [Crossref] [PubMed]
- Bhargava R, Hahn G, Hirsch W, Kim MJ, Mentzel HJ, Olsen OE, Stokland E, Triulzi F, Vazquez E. Contrast-enhanced magnetic resonance imaging in pediatric patients: review and recommendations for current practice. Magn Reson Insights 2013;6:95-111. [Crossref] [PubMed]
- Patel KB, Poplawski MM, Pawha PS, Naidich TP, Tanenbaum LN. Diffusion-weighted MRI "claw sign" improves differentiation of infectious from degenerative modic type 1 signal changes of the spine. AJNR Am J Neuroradiol 2014;35:1647-52. [Crossref] [PubMed]
- Dumont RA, Keen NN, Bloomer CW, Schwartz BS, Talbott J, Clark AJ, Wilson DM, Chin CT. Clinical Utility of Diffusion-Weighted Imaging in Spinal Infections. Clin Neuroradiol 2019;29:515-22. [Crossref] [PubMed]
- Özdemir O, Metin Y, Metin NO, Küpeli A, Kalcan S, Taşçı F. Contribution of diffusion-weighted MR imaging in follow-up of inflammatory appendiceal mass: Preliminary results and review of the literature. Eur J Radiol Open 2016;3:207-15. [Crossref] [PubMed]
- Gordon Y, Partovi S, Müller-Eschner M, Amarteifio E, Bäuerle T, Weber MA, Kauczor HU, Rengier F. Dynamic contrast-enhanced magnetic resonance imaging: fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc Diagn Ther 2014;4:147-64. [Crossref] [PubMed]
- Jans L, De Coninck T, Wittoek R, Lambrecht V, Huysse W, Verbruggen G, Verstraete K. 3 T DCE-MRI assessment of synovitis of the interphalangeal joints in patients with erosive osteoarthritis for treatment response monitoring. Skeletal Radiol 2013;42:255-60. [Crossref] [PubMed]
- Liao D, Xie L, Han Y, Du S, Wang H, Zeng C, Li Y. Dynamic contrast-enhanced magnetic resonance imaging for differentiating osteomyelitis from acute neuropathic arthropathy in the complicated diabetic foot. Skeletal Radiol 2018;47:1337-47. [Crossref] [PubMed]
- Granger DN, Rodrigues SF. Microvascular Responses to Inflammation. In: Parnham MJ, editor. Compendium of Inflammatory Diseases. Basel: Springer, 2016.
- Ertugrul BM, Savk O, Ozturk B, Cobanoglu M, Oncu S, Sakarya S. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit 2009;15:CR307-12.
- van Asten SA, Jupiter DC, Mithani M, La Fontaine J, Davis KE, Lavery LA. Erythrocyte sedimentation rate and C-reactive protein to monitor treatment outcomes in diabetic foot osteomyelitis. Int Wound J 2017;14:142-8. [Crossref] [PubMed]
- Boulton AJ. The pathway to foot ulceration in diabetes. Med Clin North Am 2013;97:775-90. [Crossref] [PubMed]
- Hulsen DJW, Mitea C, Arts JJ, Loeffen D, Geurts J. Diagnostic value of hybrid FDG-PET/MR imaging of chronic osteomyelitis. Eur J Hybrid Imaging 2022;6:15. [Crossref] [PubMed]
- Pezeshk P, Alian A, Chhabra A. Role of chemical shift and Dixon based techniques in musculoskeletal MR imaging. Eur J Radiol 2017;94:93-100. [Crossref] [PubMed]
- Mahesh M. The Essential Physics of Medical Imaging, Third Edition. Med Phys 2013;40:077301.
- Özgen A. The Value of the T2-Weighted Multipoint Dixon Sequence in MRI of Sacroiliac Joints for the Diagnosis of Active and Chronic Sacroiliitis. AJR Am J Roentgenol 2017;208:603-8. [Crossref] [PubMed]
- Grimm A, Meyer H, Nickel MD, Nittka M, Raithel E, Chaudry O, Friedberger A, Uder M, Kemmler W, Quick HH, Engelke K. Evaluation of 2-point, 3-point, and 6-point Dixon magnetic resonance imaging with flexible echo timing for muscle fat quantification. Eur J Radiol 2018;103:57-64. [Crossref] [PubMed]
- Guerini H, Omoumi P, Guichoux F, Vuillemin V, Morvan G, Zins M, Thevenin F, Drape JL. Fat Suppression with Dixon Techniques in Musculoskeletal Magnetic Resonance Imaging: A Pictorial Review. Semin Musculoskelet Radiol 2015;19:335-47. [Crossref] [PubMed]
- Low RN, Austin MJ, Ma J. Fast spin-echo triple echo dixon: Initial clinical experience with a novel pulse sequence for simultaneous fat-suppressed and nonfat-suppressed T2-weighted spine magnetic resonance imaging. J Magn Reson Imaging 2011;33:390-400. [Crossref] [PubMed]
- Komarraju A, Chhabra A. Advanced Cross-Sectional Radiology-Ultrasound, Computed Tomography and Magnetic Resonance Imaging of the Diabetic Foot. In: The Foot in Diabetes. Hoboken, NJ, USA: Wiley, 2020:169-85.
- Donovan A, Schweitzer ME. Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics 2010;30:723-36. [Crossref] [PubMed]
- Griffith JF, Yeung DK, Chow SK, Leung JC, Leung PC. Reproducibility of MR perfusion and (1)H spectroscopy of bone marrow. J Magn Reson Imaging 2009;29:1438-42. [Crossref] [PubMed]
- Li X, Kuo D, Schafer AL, Porzig A, Link TM, Black D, Schwartz AV. Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging 2011;33:974-9. [Crossref] [PubMed]
- Li X, Ma BC, Bolbos RI, Stahl R, Lozano J, Zuo J, Lin K, Link TM, Safran M, Majumdar S. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging 2008;28:453-61. [Crossref] [PubMed]
- Zhang J, Cheng K, Ding Y, Liang W, Ding Y, Vanel D, Cheng X. Study of single voxel 1H MR spectroscopy of bone tumors: differentiation of benign from malignant tumors. Eur J Radiol 2013;82:2124-8. [Crossref] [PubMed]
- Qi ZH, Li CF, Li ZF, Zhang K, Wang Q, Yu DX. Preliminary study of 3T 1H MR spectroscopy in bone and soft tissue tumors. Chin Med J (Engl) 2009;122:39-43.
- Amar M, Ghasi RG, Krishna LG, Khanna G. Proton MR spectroscopy in characterization of focal bone lesions of peripheral skeleton. Egypt J Radiol Nucl Med 2019;50:91.
- Subhawong TK, Wang X, Durand DJ, Jacobs MA, Carrino JA, Machado AJ, Fayad LM. Proton MR spectroscopy in metabolic assessment of musculoskeletal lesions. AJR Am J Roentgenol 2012;198:162-72. [Crossref] [PubMed]
- Yuen MK, Xiao L. State-of-the-art Musculoskeletal Magnetic Resonance Imaging: Technical Review. Hong Kong Journal of Radiology 2017;20:6-16.
- Mohindra N, Neyaz Z. Magnetic resonance sequences: Practical neurological applications. Neurol India 2015;63:241-9. [Crossref] [PubMed]
- Amornsiripanitch N, Bickelhaupt S, Shin HJ, Dang M, Rahbar H, Pinker K, Partridge SC. Diffusion-weighted MRI for Unenhanced Breast Cancer Screening. Radiology 2019;293:504-20. [Crossref] [PubMed]
- Perera Molligoda Arachchige AS. What must be done in case of a dense collection? Radiol Med 2021;126:1657-8. [Crossref] [PubMed]
- Baba A, Kurokawa R, Kurokawa M, Ota Y, Srinivasan A. Dynamic Contrast-Enhanced MRI Parameters and Normalized ADC Values Could Aid Differentiation of Skull Base Osteomyelitis from Nasopharyngeal Cancer. AJNR Am J Neuroradiol 2023;44:74-8. [Crossref] [PubMed]
- Allam MFAB, El-Sherif AMH, Helmy AH, Ali Abdelgawad E, Mohammad SSM, Abdel-Rahman AM. The utility of chemical shift imaging and related Dixon images in evaluation of bone marrow edema-like changes in diabetic foot. Egypt J Radiol Nucl Med 2023;54:76.


