
A case of early multifocal posttransplant lymphoproliferative disease in an adult after liver transplantation and literature analysis
Introduction
Posttransplant lymphoproliferative disorder (PTLD) is a rare postoperative complication that can occur after solid organ or allogeneic hematopoietic stem cell transplantation (HSCT) (1). It is characterized by immune dysfunction, which leads to abnormal proliferation of lymphocytes. The incidence of PTLD varies widely among patients who receive organ transplants, with the highest incidence rates reported in those with combined organ and small intestine transplants (11–33%), followed by lung transplants (2–9%), heart transplants (2–6%), and kidney transplants (1–3%). The incidence is lowest in those with liver transplants and HSCT (1–2%) (1-4). In liver transplantation (LT), PTLD tends to occur later, with an average age of onset of 7.2 years (5).
This report presents the first reported case of early-onset PTLD involving multiple organs, including the liver, spleen, stomach, and abdominal wall, after adult LT. Ultimately, the patient died of multiple organ failure 7 months after operation. We hope the reporting of this case and a discussion of the related literature can improve the understanding of this disease, enhance diagnostic accuracy, and help inform clinical treatment.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the relevant institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
On December 21, 2021, a 51-year-old male patient underwent orthotopic LT due to decompensated alcoholic cirrhosis. No significant abnormalities, such as those related to anti-Epstein-Barr virus (EBV), immunoglobulin G (IgG), and liver function, were found in the preoperative tests of the donor. He received antirejection therapy, liver preservation treatment, and other necessary interventions postoperatively. Subsequent routine follow-up examinations showed no significant abnormalities. However, a week after, the patient developed a cough and sputum after catching a cold, along with symptoms of tightness of breath after exercise and wheezing. A history of smoking and alcohol consumption for over 20 years was recorded. The patient was then admitted to the First Hospital of Shanxi Medical University for further evaluation. In laboratory tests, the cancer antigen 19-9 (CA19-9) level was around 30.63 U/mL (reference range, 0–23 U/mL), and the lymphocyte percentage was 2.4% (reference range, 20–50%).
On computed tomography (CT) and magnetic resonance imaging (MRI), the wall of the lesser curvature in the gastric cardia appeared thickened, measuring approximately 1.8 cm at its thickest point. This region displayed hyperintensity on diffusion-weighted imaging (DWI) and hypointensity on apparent diffusion coefficient (ADC) mapping. Additionally, multiple circular nodules were identified in the liver and spleen parenchyma, with the largest measuring approximately 1.8 cm × 2.4 cm × 2.1 cm in size (measured as the upper-to-lower diameters × anterior-to-posterior diameter × left-to-right diameter). These nodules exhibited slightly low density on CT, hypointensity on T1-weighted imaging (T1WI), hyperintensity on T2-weighted imaging (T2WI), and hyperintensity on DWI. After injection of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) contrast agent, the lesions demonstrated mild enhancement, which was followed by sustained enhancement in the venous and delayed phases. Contrast uptake was observed partly in the liver lesions in the hepatobiliary phase. Furthermore, multiple long T1 and T2 signal shadow-like circular nodules were observed subcutaneously in the liver hilum, retroperitoneum, and abdominal wall. These nodules exhibited hyperintensity on DWI and hypointensity on ADC. Mild and inhomogeneous enhancement was observed in the early phase of enhanced scanning, with continuous enhancement in the venous phase and delayed phase (Figure 1). The largest nodule measured approximately 3.3 cm × 2.8 cm × 2.8 cm in size (measured as the upper-to-lower diameter × anterior-to-posterior diameter × left-to-right diameter). Contrast-enhanced ultrasound was used to confirm the homology of nodules in the liver and spleen. Moreover, ultrasonography also revealed multiple enlarged lymph nodes in the left submandibular gland, left neck, and right groin area (Figure 2), with the largest one located in the left neck and measuring approximately 4.6 cm × 2.3 cm in size.


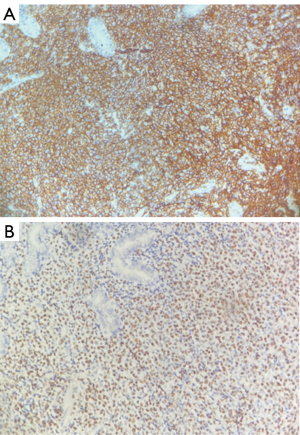
The immunohistochemical staining of the specimen from the gastroesophageal junction showed positivity for CD20 and EBV-encoded small RNA (EBER) (Figure 3). Leukocyte common antigen (LCA), vimentin, CD21, B-cell lymphoma-2 (Bcl-2), multiple myeloma oncogene 1 (MUM1), paired box gene 5 (PAX5), and CD38 were all positive. Creatine kinase (CK), CD10, BL-6, CD30, and CD5 all show negative expression. CD3 demonstrated weak positivity, while Ki-67 was strongly positive, with a 60% or higher proliferation index. Cellular-myelocytomatosis viral oncogene (C-myc) was expressed in approximately 20% of the cells. These findings suggest the presence of EBV-related non-Hodgkin diffuse large B-cell lymphoma with plasma cell hyperplasia.
Following the patient’s PTLD diagnosis, administration of tacrolimus tablets was reduced to 0.5 mg twice daily, and methylprednisolone was discontinued. Other treatment plans remained unchanged. After the patient’s symptoms improved, the patient refused further hospital treatment and left on his own accord after signing a consent form for discontinuing treatment. He was discharged from the hospital, maintained the current treatment plan, and continued to be closely monitored as an outpatient. Shortly after returning home, the patient developed symptoms of cough and wheezing. Unfortunately, the patient died at a local hospital 1 month later due to multiple organ failure.
Discussion
LT can lead to several complications, including tumor recurrence, liver failure due to rejection, biliary obstruction, graft-versus-host disease, and infection (6). PTLD is a rare complication of LT that affects approximately 2% of adults, with 13.3% of patients experiencing multifocal disease (7). PTLD can occur either early (<1 year after transplantation) or late (≥1 year after transplantation), and it is classified into five major histological forms according to the 2016 World Health Organization classification: plasmacytic hyperplasia PTLD, infectious mononucleosis PTLD, florid follicular hyperplasia PTLD, polymorphic PTLD, and monomorphic PTLD (B- and T-/natural killer-cell types) and classical Hodgkin lymphoma PTLD.
In 1968, Doak et al. were first to propose the existence of PTLD when it developed in patients with renal transplantation it (8). The etiology and pathogenesis of PTLD remain unclear. However, common risk factors include recipient EBV negativity, donor EBV positivity, patient age, intensity of immunosuppression, and the time elapsed after transplantation (9). Clinical manifestations of PTLD vary greatly, but general symptoms include fever, emaciation, night sweats, fatigue, and lymph node enlargement. The incidence of PTLD is relatively insidious, with a high rate of missed diagnosis and misdiagnosis. Delayed treatment may lead to patient death.
To identify early-onset PTLD cases in adult LT patients, we searched relevant literature using the keywords “lymphoproliferative disease”, “PTLD”, “adult”, “liver transplantation”, and “early-onset” in self-built databases of the China National Knowledge Infrastructure (CNKI), PubMed, and Google platforms, among others until January 2023. We excluded patients with incomplete case data, and ultimately identified eight eligible patients (Table 1). In most early-onset PTLD cases (14,15), immunohistochemistry of patient tissue showed positivity for EBV and CD20, and transplanted organs were commonly affected. This may be due to the loss of supervision of donor-derived lymphocytes and their malignant hyperplasia in the case of strong immunosuppression (14). In our case, the involvement of other organs of the digestive tract, abdominal wall, and peripheral lymph nodes was atypical for early-onset PTLD. We believe this may be due to the proliferation of EBV-positive cells escaping from the recipient’s immune system (14).
Table 1
| Variables | Case 1 (10) | Case 2 (11) | Case 3 (11) | Case 4 (12) | Case 5 (13) | Case 6 (14) | Case 7 (14) |
|---|---|---|---|---|---|---|---|
| Age (years), gender | 49, F | 55, M | 38, M | 43, M | 54, M | 47, M | 42, M |
| Clinical presentation | Malaise, pruritis, poor appetite, and clay-colored stool | Fever, jaundice | Fever, hepatic dysfunction | Long-term biliary tract l infection | Fever, jaundice | – | – |
| Time from transplant (months) | 9 | 6 | 5 | 7 | 4 | 7 | 11 |
| Site of disease | Porta hepatis | Porta hepatis | Porta hepatis | Porta hepatis | Porta hepatis | Porta hepatis | Porta hepatis |
| Imaging and autopsy | CT scans: a 5.0 cm × 4.8 cm × 6.0 cm soft tissue mass | CT and ultrasound: a hilar mass involving the portal vein and biliary tract | CT and ultrasound: a hilar mass involving the hepatic artery, portal vein, and biliary tract | CT: para-arterial lymph nodes 3.0 cm × 2.0 cm × 2.0 cm in size | Autopsy: inflammatory exudation in the portal vein space with a large number of plasma cells | Hilar biliary stenosis | – |
| EBV positivity | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
| Immunohistochemistry | CD3+ | CD45RO+/CD20− | CD45RO−/CD20+ | CD45RO−/CD20+ | CD3+/CD43+ | CD20+ | CD20+/BCL6+/CD38+ |
| Diagnosis | Monomorphic PTLD (T-cell types) | Monomorphic PTLD (T-cell types) | Monomorphic PTLD (B-cell types) | Plasmacytic hyperplasia PTLD | Plasmacytic hyperplasia PTLD | Monomorphic PTLD (B-cell types) | Monomorphic PTLD |
| Treatment | Immunosuppression; antiviral therapy; radiotherapy | Immunosuppression; antiviral therapy | Immunosuppression; antiviral therapy; CHOP; rituximab | Immunosuppression; CHOP; rituximab | Immunosuppression; antiviral therapy | Rituximab; CVP; CHOP | Rituximab; CHOP |
| Follow-up (months) | 19 | 6 | 4 | 11 | 0.5 | 3 | 5 |
| Clinical outcome | Alive | Dead | Dead | Alive | Dead | Dead | Dead |
| Cause of death | – | Lymphoma relapse | Respiratory failure | – | Generalized infection | Blood poisoning | Massive hemobilia |
PTLD, posttransplant lymphoproliferative disorder; LT, liver transplantation; F, female; M, male; CT, computed tomography; EBV, Epstein-Barr virus; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone.
Although the morbidity and mortality rates of PTLD have decreased over recent years, there are still no clear consensus guidelines for its treatment. Currently, the first step in treating both early- and late-stage PTLD is reducing or even stopping immunosuppressants, but only about half of patients respond to this therapy (16,17). If reducing immunosuppression fails, rituximab for CD20 is recommended for patients with early-onset PTLD. Studies have shown that the total response rate of rituximab as PTLD treatment may be as high as 44% (18), with good safety. To treat patients with late-onset PTLD, chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) is often used. For patients with a single-focal PTLD, surgical excision and/or radiotherapy may be used to reduce immunosuppression.
In order to further prevent PTLD, several measures should be taken, including monitoring primary EBV infection and the reactivation of EBVs after transplantation through polymerase chain reaction testing. Studies have shown that patients with serologically negative EBV may experience a reduction in immunosuppressant use when an increase in EBV load is detected (19). The standardized management of LT patients can effectively reduce the incidence of PTLD and enable early detection of PTLD lesions.
Conclusions
This is the first report of a case of early multifocal PTLD after adult LT and thus may be a valuable reference. In the future, with better understanding of the disease and improved diagnosis, the incidence of PTLD may increase. Close and long-term follow-up is necessary in managing patients with PTLD.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-566/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singavi AK, Harrington AM, Fenske TS. Post-transplant lymphoproliferative disorders. Cancer Treat Res 2015;165:305-27. [Crossref] [PubMed]
- Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, Marcus R, Parameshwar J, Ramsay A, Newstead CHaemato-oncology Task Force of the British Committee for Standards in Haematology and British Transplantation Society. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol 2010;149:675-92. [Crossref] [PubMed]
- Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol 2005;56:155-67. [Crossref] [PubMed]
- Faull RJ, Hollett P, McDonald SP. Lymphoproliferative disease after renal transplantation in Australia and New Zealand. Transplantation 2005;80:193-7. [Crossref] [PubMed]
- Cruz RJ Jr, Ramachandra S, Sasatomi E, DiMartini A, de Vera M, Fontes P, Hughes C, Humar A. Surgical management of gastrointestinal posttransplant lymphoproliferative disorders in liver transplant recipients. Transplantation 2012;94:417-23. [Crossref] [PubMed]
- Zhang Q, Chen H, Chen XG, Wang Y, Shen ZY. Prognostic factors affecting survival after liver transplantation for hepatocellular carcinoma patients with hepatitis B virus infection and cirrhosis. Practical Journal of Organ Transplantation 2015;3:215-21. (Electronic Version).
- Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 2004;4:222-30. [Crossref] [PubMed]
- Doak PB, Montgomerie JZ, North JD, Smith F. Reticulum cell sarcoma after renal homotransplantation and azathioprine and prednisone therapy. Br Med J 1968;4:746-8. [Crossref] [PubMed]
- Kamdar KY, Rooney CM, Heslop HE. Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant 2011;16:274-80. [Crossref] [PubMed]
- Koffman BH, Kennedy AS, Heyman M, Colonna J, Howell C. Use of radiation therapy in posttransplant lymphoproliferative disorder (PTLD) after liver transplantation. Int J Cancer 2000;90:104-9. [Crossref] [PubMed]
- Huai MS, Gao W, Wu D, Zhang QS, Wang ZL, Zheng H. Occurrence of hilar malignant lymphoma after liver transplantation: A report of two cases. Chinese Hepatology 2007;13-6.
- Zheng H, Huai MS, Wu D, Gao W, Zhang QS, Wang ZL, Shen ZY. Liver-localized lymphoproliferative disease following liver transplantation-Report of 3 cases. Chinese Journal of Organ Transplantation 2007;28:460-2.
- Ziarkiewicz-Wróblewska B, Górnicka B, Ołdakowska U, Suleiman W, Pratnicki A, Szymańska-Giemza O, Ziołkowski J, Senatorski G, Pacholczyk M, Łagiewska B, Rowiński W, Paczek L, Wasiutyński A. Plasmacytic hyperplasia--the early form of posttransplant lymphoproliferative disorder--with atypical morphology and clinical course in patient after liver transplantation: a case report. Transplant Proc 2003;35:2320-2. [Crossref] [PubMed]
- Avolio AW, Agnes S, Barbarino R, Magalini SC, Frongillo F, Pagano L, Larocca LM, Pompili M, Caira M, Sollazzi L, Castagneto M. Posttransplant lymphoproliferative disorders after liver transplantation: analysis of early and late cases in a 255 patient series. Transplant Proc 2007;39:1956-60. [Crossref] [PubMed]
- Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, Ansell SM, Gores GJ, Stegall MD, McGregor CG. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation 2005;79:244-7. [Crossref] [PubMed]
- Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep 2013;8:173-83. [Crossref] [PubMed]
- Zimmermann H, Trappe RU. EBV and posttransplantation lymphoproliferative disease: what to do? Hematology Am Soc Hematol Educ Program 2013;2013:95-102. [Crossref] [PubMed]
- Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood 2006;107:3053-7. [Crossref] [PubMed]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9:S1-155. [Crossref] [PubMed]


