
Preoperative metabolic parameters of 18F-FDG PET/CT are associated with TNM stage and prognosis of colorectal cancer patients
Introduction
Colorectal cancer (CRC) is the second most common cancer in women and the third in men worldwide, and despite advances in treatment, CRC is still the third most frequent cause of cancer-related death (1). The traditional and current guidelines for survival prediction and surveillance recommendations for CRC remain the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) tumor/node/metastasis (TNM) anatomical classification (2). Although the five-year survival rate for CRC patients at the early stage (stage I and II) is above 60%, CRC with metastatic disease remains challenging. More than 50% of patients are diagnosed at an advanced stage (stage III and IV) associated with metastasis to lymph nodes or distant organs, the 5-year survival rate drops to 10% (3). Surgical resection is currently the primary treatment for CRC patients who have not spread to distant sites. In inoperable patients, radiotherapy, chemotherapy, immunotherapy, and targeted therapy can be selected on the basis of the actual situation of the patients (4). However, the postoperative pathological TNM (pTNM) stage may be different from the preoperative clinical TNM (cTNM) stage, according to the AJCC Cancer Staging Classification (2). Therefore, an effective and non-invasive diagnostic tool is needed to assess CRC staging and predict prognosis accurately, as that help choosing patient-specific treatment strategies preoperatively to reduce tumor progression and improve survival.
2-deoxy-2[18F]fluoro-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) is an important molecular imaging modality that is used for diagnosing and performing TNM staging in CRC, which could accurately access the preoperative tumor localization in early-stage patients or lymph node and distant statuses in advanced-stage patients (5). 18F-FDG PET/CT is better than conventional imaging, such as serial enhanced CT from lung base to the pelvis, for the staging and follow-up of CRC in all localities, with higher sensitivity, specificity, and accuracy (6,7). PET metabolic parameters, including maximum and minimum standardized uptake value (SUVmax, SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), are correlated with the clinicopathological characteristics and prognosis in several tumors, such as oral squamous cell cancer, esophageal cancer, and non-small-cell lung cancer (8-10). The SUVmax only reflects the highest extent of glucose utilization in the tumor, the volumetric parameters such as MTV and TLG are promising semiquantitative parameters to assess the metabolic activity of the whole tumor, which could provide additional information on intratumoral biological variation (11).
Therefore, the objective of this study was to investigate the application value of metabolic parameters of 18F-FDG PET/CT in assessments of TNM staging of CRC patients, to analyze the relationship of FDG metabolic parameters with TNM stages and serological biomarkers in CRC, and to predict the prognosis of CRC patients. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-966/rc).
Methods
Patients and clinicopathological features, and follow up
From September 2016 to August 2022, a total of 132 patients with CRC proved by surgical pathology who underwent preoperative 18F-FDG PET/CT examination for clinical staging in our hospital were retrospectively and consecutively included in this cross-sectional study. The major inclusion criteria were as follows: (I) surgical histopathology-proven CRC; (II) PET/CT scan was performed within 2 weeks prior to surgical resection of CRC. Exclusion criteria were: (I) patients who received neoadjuvant treatment (such as chemotherapy and/or radiotherapy) before the surgery; (II) less than 18 years old; (III) patients with other malignancies. The flowchart of selection criteria is shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Friendship Hospital, Affiliated to Capital Medical University (No. 2023-P2-013-01), and the requirement for informed consent was waived because of the retrospective nature of the study. The data of all patients were anonymized in this paper.
Clinicopathological information was obtained from the medical records. General information such as gender and age, and serological biomarkers such as serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) levels, were obtained 2 weeks within the PET/CT scan. Primary tumor information was generated from the surgical pathology reports, including tumor location, tumor size, differentiation, vascular invasion, lymphovascular invasion, pathological T (pT) stage, and pN stage. The metastasis status (M stage) was accessed by the preoperative imaging evaluation such as CT, MRI, and 18F-FDG PET/CT. The pT, pN, and M stage, and cancer TNM stage are defined according to the AJCC 8th edition staging system (2).
Postoperative follow-up data were collected from medical records and telephone consultations. Imaging evaluations such as chest-abdominal-pelvis CE-CT, plain CT, pelvis magnetic resonance imaging (MRI), or whole-body 18F-FDG PET/CT were performed regularly after surgery (once every 3–6 months). Progression-free survival (PFS) was defined as the time from the operative PET/CT scan to disease progression accessed by imaging evaluation. All patients were followed up for at least 1 year. Patients without progression were censored at the date of the last follow-up visit.
PET/CT imaging
All PET/CT examinations were performed using a Biograph mCT S64 (Siemens Healthineers Medical Solutions), which collects PET and CT data simultaneously. All patients were required to fast for more than 6 h prior to PET/CT examinations, intravenous injection of 18F-FDG (approximately 4.4 MBq/kg) was performed when the blood glucose level was <11.1 mmol/L. The PET/CT scan started 1h after the tracer injection. Thereafter, the patient was asked to void and subsequently was placed in a supine position with the arms up in the scanner. Patients were scanned from the skull base to the upper one-third of the femur. The CT scan (120 kV, 200 mA, and slice thickness of 3 mm) was performed for attenuation correction and anatomical localization. The following PET scan was performed in a three-dimensional (3D) mode (2.5 min/bed position, 6–8 beds). PET images were reconstructed using the ordered subset expectation maximization iterative method with CT data for attenuation correction.
Image analysis
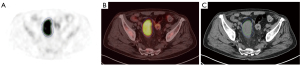
Two experienced nuclear medicine physicians with more than 5-year experience in PET/CT diagnosis, who were masked to the medical history of the participants, independently reviewed the PET/CT images on a dedicated workstation (syngo MultiModality Workplace, Siemens, Erlangen, Germany). Any disagreement would be discussed with another senior expert (experience >15 years) till an agreement was reached. The volume of interest (VOI) of the primary tumor was delineated using 3D slicer, a free open-source platform for medical image computing. To improve the accuracy and repeatability of imaging analysis, the VOI was drawn in two steps for each patient by two experienced nuclear medicine physicians. Firstly, a primary VOI around the margin of the primary tumor was delineated in 3D on the PET image manually. The inner and outer edges of the tumor were mainly determined by PET image, with consideration of CT image. The boundary between tumor and normal physiological uptake was carefully determined. Then a final secondary VOI based on SUVmax (40%) was automatically delineated, as the cut-off is commonly used (12). An example of the VOI is illustrated in Figure 2. The SUVmax, TLG, and MTV of the secondary VOI were obtained and recorded for FDG semi-quantitative analysis. The mean liver SUV (SUVliver) was determined by the average of SUVmean of 3 sphere VOIs approximately 3 cm in diameter on the normal right and left liver. Tumor-to-liver standardized uptake value ratio (SUVR) of the primary tumors was computed as the ratio of the tumor SUVmax to SUVliver.
Statistical analysis
Data were analyzed with Madcalc software (version 19.0), and figures were generated using GraphPad Prism 8 (GraphPad Software, San Diego, CA) and R 4.0.2 software (Bell Laboratories, Holmdel, NJ, USA). Continuous variables with a skewed distribution were reported as median [interquartile range (IQR)], and categorical variables by numbers (percentages). Continuous variables were compared with Mann-Whitney U test, and categorical variables with the two-sided Pearson Chi-square test. Each continuous parameter that was significantly associated with the advanced TNM stage (stage III–IV) was analyzed as categorical variables using cut-points determined by receiver operator characteristic (ROC) curve analysis, while others were analyzed as categorical variables using the median as cut-points. The possible risk factors associated with the advanced TNM stage were investigated by the univariate logistic regression analysis to estimate the odds ratio (OR) and 95% confidence interval (CI), using the backward stepwise method. Then the factors with P<0.05 were included in the multivariate logistic regression analysis (backward stepwise method). Factors that remained significant at P<0.05 were considered to be independent predictors of the advanced TNM stage. PFS was analyzed using Kaplan-Meier curves and Log-rank test. All tests were two-sided with a significance level of P<0.05.
Results
Patients characteristics
A total of 132 patients (84 male (63.6%)] conformed to our inclusion criteria were included in the study, with a median age of 65 [interquartile range (IQR), 58–74] years. The primary tumor of 36 (27.3%) patients was localized in the right hemi-colon. According to primary tumor pathology, the moderately-differentiated tumor was the most common histologic grade (86.4%), while well- and poorly-differentiated tumors represented 3.8% and 9.8%, respectively. The majority of patients were pT3 (64.4%), pN1–2 (53.8%), M0 (73.5%), and TNM III–IV (59.8%) stage. There were only 122 patients with information on vascular invasion status and 115 patients with lymphovascular invasion status, as the other data were not available in some early pathological reports. And 48 (39.3%) of 122 patients had vascular invasion and 33 (28.7%) of 115 patients had lymphovascular invasion. The clinicopathological characteristics of CRC patients are exhibited in Table 1.
Table 1
| Characteristics | Value |
|---|---|
| General information | |
| Age, M [IQR], years | 65 [58–74] |
| Gender, n (%) | |
| Male | 84 (63.6) |
| Female | 48 (36.4) |
| Primary tumor information, n (%) | |
| Tumor location | |
| Right | 36 (27.3) |
| Left | 96 (72.7) |
| Tumor size, M (IQR), (cm) | 5.0 (3.5–6.0) |
| Differentiation | |
| Well | 5 (3.8) |
| Moderately | 114 (86.4) |
| Poorly | 13 (9.8) |
| Vascular invasion | |
| Yes | 48 (39.3) |
| No | 74 (60.7) |
| Lymphovascular invasion | |
| Yes | 33 (28.7) |
| No | 82 (71.3) |
| Staging, n (%) | |
| pT stage | |
| T1 | 3 (2.3) |
| T2 | 9 (6.8) |
| T3 | 85 (64.4) |
| T4 | 35 (26.5) |
| pN stage | |
| N0 | 61 (46.2) |
| N1 | 45 (34.1) |
| N2 | 26 (19.7) |
| M stage | |
| M0 | 97 (73.5) |
| M1 | 35 (26.5) |
| TNM stage | |
| I | 10 (7.6) |
| II | 43 (32.6) |
| III | 44 (33.3) |
| IV | 35 (26.5) |
TNM stages are defined according to the American Joint Committee on Cancer 8th edition staging system. M, median; IQR, interquartile range; TNM, tumor/node/metastasis.
Correlation between metabolic parameters and advanced TNM stage in CRC
To explore the relationship between metabolic parameters and tumor stage, we defined early and advanced groups according to the TNM (stage I–II vs. III–IV), pT (stage 1–3 vs. 4), pN (stage 0 vs. 1–2), and M (stage 0 vs. 1) stage based on prior similar studies (13,14), respectively.
The results showed that metabolic parameters of MTV, TLG and serological biomarker of CEA, and CA19-9 in the advanced TNM stage group (stage III–IV) were significantly higher than the early group (P=0.005, 0.016, 0.0003, 0.033, respectively). Besides, larger tumor size, higher SUVR, MTV, and TLG were significantly correlated with advanced pT stage (stage 4), and higher MTV, and TLG were significantly correlated with advanced pN stage (stage 1–2) (P<0.05), while no metabolic parameters were significantly correlated with metastasis status (P>0.05). Higher CEA and CA19-9 levels were significantly correlated with advanced pT, pN stage, and metastasis status (P<0.05) (Tables 2,3, Figures 3,4).
Table 2
| Characteristics | TNM stage | P value | |
|---|---|---|---|
| I–II (n=53) | III–IV (n=79) | ||
| General information | |||
| Age, M [IQR], years | 65 [55.5–75.5] | 66 [59–73] | 0.424 |
| Gender, n (%) | 0.639 | ||
| Female | 18 (13.6) | 30 (22.7) | |
| Male | 35 (26.5) | 49 (37.1) | |
| Primary tumor information | |||
| Tumor location, n (%) | 0.168 | ||
| Right | 11 (8.3) | 25 (18.9) | |
| Left | 42 (31.8) | 54 (40.9) | |
| Tumor size, M (IQR), cm | 4.5 (3.1–5.6) | 5.0 (3.5–6.5) | 0.237 |
| Metabolic parameters, M (IQR) | |||
| SUVmax (g/cm3) | 15.7 (11.6–19.8) | 14.9 (11.2–19.8) | 0.952 |
| SUVR | 4.6 (3.3–6.7) | 5.0 (3.5–6.6) | 0.797 |
| MTV (g/cm3) | 10.4 (5.8–22.8) | 18.8 (10.7–28.2) | 0.005** |
| TLG (g) | 81.3 (50.1–197.2) | 141.6 (87.5–265.9) | 0.016* |
| Serum tumor marker, M (IQR) | |||
| CEA (ng/mL) | 3.32 (1.67–4.79) | 6.06 (2.79–17.84) | 0.0003*** |
| CA19-9 (U/mL) | 15.30 (5.26–55.96) | 16.80 (9.20–69.10) | 0.033* |
*, P<0.05; **, P<0.01; ***, P<0.001. TNM, tumor/node/metastasis; M, median; IQR, interquartile range; SUVmax, maximum standard uptake value; SUVR, tumor-to-liver standardized uptake value ratio; MTV, metabolic tumor volume; TLG, total lesion glycolysis; CEA, serum carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Table 3
| Characteristics | pT stage | pN stage | M stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–3 (n=97) | 4 (n=35) | P | 0 (n = 61) | 1–2 (n=71) | P | 0 (n=97) | 1 (n=35) | P | |||
| General information | |||||||||||
| Gender | 0.127 | 0.996 | 0.352 | ||||||||
| Female | 39 (29.5) | 9 (6.8) | 22 (16.7) | 26 (19.7) | 33 (25.0) | 15 (11.4) | |||||
| Male | 58 (43.9) | 26 (19.7) | 39 (29.5) | 45 (34.1) | 64 (48.5) | 20 (15.2) | |||||
| Age (years) | 66 (57–75.5) | 64 (59–73) | 0.470 | 65 (57–75.5) | 65 (59–73) | 0.862 | 65 (57–75.5) | 65 (59–73.0) | 0.777 | ||
| Primary tumor information | |||||||||||
| Tumor location | 0.277 | 0.069 | 0.809 | ||||||||
| Right | 24 (18.2) | 12 (9.1) | 12 (9.1) | 24 (18.2) | 27 (20.5) | 9 (6.8) | |||||
| Left | 73 (55.3) | 23 (17.4) | 49 (37.1) | 47 (35.6) | 70 (53.0) | 26 (19.7) | |||||
| Tumor size (cm) | 4.5 (3.2–4.5) | 5.5 (4.5–6.5) | 0.020* | 4.5 (3.1–5.5) | 5.0 (3.5–6.5) | 0.079 | 5.0 (3.25–5.75) | 5.0 (3.5–7.0) | 0.490 | ||
| Metabolic parameters | |||||||||||
| SUVmax (g/cm3) | 14.3 (11.3–18.9) |
18.5 (11.8–21.9) |
0.132 | 14.7 (11.1–18.9) |
15.4 (11.4–21.2) |
0.629 | 15.7 (11.7–21.7) |
13.7 (10.4–18.8) |
0.288 | ||
| SUVR | 4.5 (3.4–6.2) | 6.1 (3.5–7.6) | 0.031* | 4.6 (3.1–6.2) | 5.0 (3.7–6.9) | 0.291 | 5.2 (3.4–7.1) | 4.2 (3.5–6.4) | 0.251 | ||
| MTV (g/cm3) | 12.1 (6.5–26.0) |
20.5 (11.6–25.1) |
0.040* | 10.4 (6.0–22.2) |
19.6 (11.6–28.7) |
0.0004*** | 14.3 (6.7–26.4) |
15.5 (10.6–24.9) |
0.402 | ||
| TLG (g) | 102.1 (57.9–207.1) |
175.2 (116.5–275.8) |
0.010* | 80.6 (50.1–188.4) |
152.5 (97.8–275.8) |
0.001** | 107.5 (61.1–231.1) |
129.8 (87.5–235.6) |
0.447 | ||
| Serum tumor markers | |||||||||||
| CEA (ng/mL) | 3.50 (2.04–7.49) |
12.89 (4.78–38.42) |
<0.0001*** | 3.44 (2.04–5.21) |
6.06 (2.77–18.95) |
0.001** | 3.88 (2.17–8.10) |
9.04 (2.92–18.95) |
0.003** | ||
| CA19-9 (U/mL) | 11.40 (5.43–23.25) |
45.40 (15.60–134.10) |
<0.0001*** | 16.00 (5.15–23.81) |
16.80 (9.91–74.20) |
0.024* | 15.30 (6.27–22.75) |
32.90 (9.91–134.10) |
0.001** | ||
Data are presented as M (IQR) or number (percentages). *, P<0.05; **, P<0.01; ***, P<0.001. SUVmax, maximum standard uptake value; SUVR, tumor-to-liver standardized uptake value ratio; MTV, metabolic tumor volume; TLG, total lesion glycolysis; CEA, serum carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; M, median; IQR, interquartile range.


Logistic regression analysis for prediction advanced TNM stage
The four continuous variables associated with the advanced TNM stage, including MTV, TLG, CEA, and CA19-9, were computed into categorical variables using cut-points determined by ROC curve analysis (MTV >6.6 cm3, P=0.005; TLG >88.5 g, P=0.016; CEA >5.84 ng/mL, P=0.0003; CA19-9 >47.2 U/mL, P=0.033, respectively), while other continuous variables were computed into categorical variables by media in the Table 1, which were similar with the previous literature (15-17). In univariate logistic regression, MTV >6.6 cm3 (OR =7.37, 95% CI: 2.71–20.01, P=0.0001), TLG >88.5 g (OR =3.51, 95% CI: 1.66–7.47, P=0.0011), CEA >5.84 ng/mL (OR =5.27, 95% CI: 2.27–12.24, P=0.0001), and CA19-9 >47.2 U/mL (OR =3.94, 95% CI: 1.39–11.17, P=0.0098) were significantly correlated with advanced TNM stage. In multivariate logistic regression, MTV >6.6 cm3 (OR =5.81, 95% CI: 2.05–16.44, P=0.0009) and CEA >5.84 ng/mL (OR =4.30, 95% CI: 1.79–10.34, P=0.0011) were significantly correlated with advanced TNM stage independently (Table 4).
Table 4
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| General information | |||||
| Age (>65 vs. ≤65 years) | 1.239 (0.617–2.490) | 0.547 | – | – | |
| Gender (female vs. male) | 1.190 (0.575–2.465) | 0.639 | – | – | |
| Primary tumor information | |||||
| Tumor location (right vs. left hemi-colon) | 0.566 (0.250–1.279) | 0.171 | – | – | |
| Tumor size (>5.0 vs. ≤5.0 cm) | 1.395 (0.677–2.875) | 0.367 | – | – | |
| Metabolic parameters | |||||
| SUVmax (>15.0 vs. ≤15.0 g/cm3) | 0.939 (0.468–1.883) | 0.859 | – | – | |
| SUVR (>4.9 vs. ≤4.9) | 1.065 (0.531–2.136) | 0.859 | – | – | |
| MTV (>6.6 vs. ≤6.6 cm3) | 7.37 (2.71–20.01) | 0.0001*** | 5.81 (2.05–16.44) | 0.0009*** | |
| TLG (>88.5 vs. ≤88.5 g) | 3.51 (1.66–7.47) | 0.0011** | – | – | |
| Serum tumor marker | |||||
| CEA (>5.84 vs. ≤5.84 ng/mL) | 5.27 (2.27–12.24) | 0.0001*** | 4.30 (1.79–10.34) | 0.0011* | |
| CA19-9 (>47.2 vs. ≤47.2 U/mL) | 3.94 (1.39–11.17) | 0.0098** | – | – | |
*, P<0.05; **, P<0.01; ***, P<0.001. TNM, tumor/node/metastasis; OR, odds ratio; CI, confidence interval; SUVmax, maximum standard uptake value; SUVR, tumor-to-liver standardized uptake value ratio; MTV, metabolic tumor volume; TLG, total lesion glycolysis; CEA, serum carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Prognosis value of metabolic parameters and TNM stage for PFS
The mean postoperative follow-up duration was 27.1 (IQR, 15.6–47.4; range, 1.9–73.7) months. Seven patients developed disease progression in 1 year, while other patients were followed up for at least 1 year. A total of 119 patients enrolled, and 38 (31.9%) patients with disease progress were documented during the follow-up. The prognostic analysis demonstrated that MTV >6.6 cm3 was the only metabolic parameter significantly associated with worse PFS (P=0.032). Besides, the older age (>65 years), vascular invasion, advanced TNM (stage III–IV), pT (stage 4), pN (stage 1–2), M (stage 1) stage, CEA >5.84 ng/mL, and CA19-9 >47.2 U/mL were significantly related to worse PFS (P=0.044, 0.034, 0.0002, 0.001, 0.008, <0.0001, 0.001, 0.0005, respectively) (Table 5, Figure 5).
Table 5
| Characteristics | Progression-free survival | |
|---|---|---|
| χ2 | P value | |
| General information | ||
| Age (>65 vs. ≤65 years) | 4.060 | 0.044* |
| Gender (female vs. male) | 0.165 | 0.684 |
| Primary tumor information | ||
| Tumor location (right vs. left hemi-colon) | 0.632 | 0.427 |
| Tumor size (>5.0 vs. ≤5.0 cm) | 1.012 | 0.314 |
| Differentiation (poor vs. well and moderate) | 0.344 | 0558 |
| Vascular invasion (yes vs. no) | 4.492 | 0.034* |
| Lymphovascular invasion (yes vs. no) | 0.710 | 0.399 |
| Staging | ||
| TNM (III–IV vs. I–II) | 13.740 | 0.0002*** |
| pT (4 vs. 1–3) | 11.277 | 0.001** |
| pN (1–2 vs. 0) | 7.028 | 0.008** |
| M (1 vs. 0) | 45.131 | <0.0001*** |
| Metabolic parameters | ||
| SUVmax (>15.0 vs. ≤15.0 g/cm3) | 0.029 | 0.864 |
| SUVR (>4.9 vs. ≤4.9) | 0.161 | 0.688 |
| MTV (>6.6 vs. ≤6.6 cm3) | 4.619 | 0.032* |
| TLG (>88.5 vs. ≤88.5 g) | 0.612 | 0.434 |
| Serum tumor marker | ||
| CEA (>5.84 vs. ≤5.84 ng/mL) | 10.643 | 0.001** |
| CA19-9 (>47.2 vs. ≤47.2 U/mL) | 12.145 | 0.0005*** |
*, P<0.05; **, P<0.01; ***, P<0.001. TNM, tumor/node/metastasis; SUVmax, maximum standard uptake value; SUVR, tumor-to-liver standardized uptake value ratio; MTV, metabolic tumor volume; TLG, total lesion glycolysis; CEA, serum carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.

Discussion
The present study evaluated the value of metabolic parameters of 18F-FDG PET/CT in predicting the TNM stage and prognosis in CRC. The results demonstrated that metabolic parameters, especially MTV, were associated with the advanced TNM stage, which was associated with patients’ PFS. MTV >6.6 cm3 was associated with worse PFS. Therefore, we conclude that the metabolic parameters derived from 18F-FDG PET/CT were promising image biomarkers to evaluate tumor TNM stage and predict the prognosis of CRC noninvasively.
The malignant tumor stage proposed by the UICC/AJCC TNM classification is a mainstay tool in the assessment of treatment effectiveness and survival in the clinical decision-making process (18). The degree of tumor progression and invasion, patient outcome, treatment allocation, as well as clinical trial enrolment, are estimated based on this staging system (19). The National Comprehensive Cancer Network (NCCN) clinical practice guidelines proposed that neoadjuvant therapy should be performed for patients with advanced-stage CRC (TNM stage III–IV) to reduce the recurrence rate and improve survival after surgery (20). Conventional tumor clinical staging modalities rely on multiple-site MRI or CT scans for the T and N stage and biopsy or clinical follow-up of suspected metastases to regional lymph nodes or distant sites for the N and M stage, which is difficult to reflect the overall tumor appearance and whole-body status in such way (21). Thus, a need for an accurate diagnosis, prognosis, and treatment guidelines has led radiologists to focus on whole-body molecular imaging to predict the TNM stage and prognosis effectively and non-invasively.
18F-FDG PET/CT is a useful imaging tool for staging, restaging, treatment response monitoring, and prognosis predicting in many tumors, including CRC (22). As the Warburg effect shows, the rapidly proliferating tumor cells need glycolysis to increase energy supply, and the increased expression of glucose transporter 1 (GLUT-1) in tumor cells increases glucose absorption (23). Thus, high levels of FDG uptake of the primary tumor before treatment were associated with more aggressive tumor biological behavior (24). Consistent with our findings, patients with advanced stage are prone to a higher 18F-FDG uptake of primary lesions. The SUVmax only reflects the highest glucose metabolism as measured in the highest pixels within a designated region of interest. But the MTV and TLG are suggested to provide more accurate prediction on the tumor burden and tumor behavior in an entire tumor mass as the MTV is defined as the volume of tumor cells with high glucose uptake, while TLG is defined according to the SUV and the volume of the tumor. However, only a few studies have accessed the correlation between the volumetric parameters and the TNM stage in CRC, as well as their predictive value in patients’ tumor progress and survival (15,25,26).
It is reported that 18F-FDG PET/CT parameters are higher in advanced-stage tumors than in early-stage tumors (15). To our knowledge, however, few previous studies have investigated the relationship of metabolic parameters in predicting the postoperative pTNM stage, the gold standard for making clinical decisions. Besides, the absence of universally accepted thresholds limits the predicted value of the TNM stage in the individual patient (15). Li et al. compared the preoperative clinical early- and advanced-stage groups based on the PET/CT in only 88 patients and suggested that higher SUVmax correlated with advanced clinical stage (stage III–IV) (27). Suzuki et al. reviewed the TNM stage of 138 patients but only found a trend in correlation with SUVmax, MTV2.5, and TLG2.5 (Spearman correlation coefficient =0.171, 0.357, 0.340, respectively, P<0.05) (15). In our study, we compared the early- and advanced-stage groups based on the postoperative pathology and follow-up, and found that metabolic parameters and serum tumor markers, especially MTV and CEA, were significantly higher in the latter group. In addition, we suggested meaningful cut-off values of MTV >6.6 cm3 and CEA >5.84 ng/mL to independently predict the advanced TNM stage (stage III–IV). The correlation between metabolic parameters of 18F-FDG PET/CT and TNM stage needs to be further investigated.
The previous studies also found a correlation among the metabolic parameters, the pT, pN, and M stage. Suzuki reported that MTV2.5 and TLG50% were reliable diagnostic biomarkers for discriminating T, N, and M stage, with a cut-off value of MTV2.5 =9.35 and 63.33 cm3, TLG50% =328.1 g, and TLG50% =94.81 g, respectively (15). Our previous small-size study demonstrated the higher SUVmax, TLG40%, and MTV40% values of the primary tumor lesion were correlated with an advanced T stage (stage 3–4), SUVmean correlated with M status, and no metabolic parameters correlated with N stage (28). However, the results of Kido et al. revealed that SUVmax, MTV 3.5, and TLG 40% correlated with the N stage (26). On the contrary, a study with a small sample size of 66 patients found that SUVmax was not related to the pT, pN, or M stage (29). Our present study included a relatively larger number of patients and found that SUVR and TLG differed between pT1–3 and pT4 groups, indicating that SUVR and TLG may be related to the depth of tumor invasion. We also found that TLG and MTV significantly differed between pN0 and pN1–2 groups, indicating that TLG and MTV may be related to the status of lymphovascular invasion. Contradictorily, the metabolic parameters including MTV and TLG were not significantly related to the status of lymphovascular invasion in our present study. This might be caused by the missing data, which was not available in some early pathological reports. Differently from some previous studies, our present study revealed that no metabolic parameters significantly differed between M0 and M1 groups, which may be caused by the biased results from the relatively small numbers of patients with distant metastasis. Further investigation is needed to explore whether MTV and TLG are related to the depth of invasion, lymphovascular invasion, and distant metastasis.
The tumor TNM classification system is the most commonly used prognostic factor in patients with CRC recommended by AJCC (30). 18F-FDG PET/CT metabolic and volumetric parameters, reflecting the glucose metabolism, have also been used to predict the prognosis of CRC patients. Shi et al. revealed that TNM stage and SUVmax were associated with survival by multivariate analysis (31). Two volumetric parameters MTV and TLG, which have already been recognized as markers of tumor aggressiveness and tumor burden, have also been found to be of prognostic significance in the previous studies (25,32). Xu et al. reported that N-stage status, and MTV40% were significantly associated with patients’ disease-free survival (14). Jo et al. reported that advanced TNM stage, high MTV, and high TLG, were associated with worse recurrence-free survival and overall survival in rectal cancer (13). Our study demonstrated that the advanced TNM stage, pT, pN, and M stage, as well as elevated MTV, were significantly associated with worse PFS, while MTV >6.6 cm3 could be an optimal cutoff value.
Limitations
Some limitations should be mentioned. Firstly, the study was limited by its retrospective design and by its relatively small sample size. Importantly, the various treatment methods after the surgery may affect the accuracy of the results. Besides, the observation time is limited. In the future, more extensive prospective studies involving many subjects are required to confirm the findings.
Conclusions
Baseline 18F-FDG PET-CT-derived parameters can serve as a noninvasive tool for preoperatively staging CRC. The metabolic and volumetric parameters, especially MTV >6.6 cm3, might be associated with the advanced TNM stage. In addition, MTV >6.6 cm3 and advanced TNM stage were associated with worse PFS.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-966/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-966/coif). All authors report that this study was funded by the National Natural Science Foundation of China (Nos. 82272034, 82001861, 82102088, and 82001860), Capital’s Funds for Health Improvement and Research (No. 2020-2-2025), and National Key Research and Development Plan (No. 2020YFC0122000). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Friendship Hospital, Affiliated to Capital Medical University (No. 2023-P2-013-01), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol 2018;25:1454-5.
- McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr Med Chem 2017;24:1537-57. [Crossref] [PubMed]
- Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev Cancer 2020;1874:188447. [Crossref] [PubMed]
- Agarwal A, Marcus C, Xiao J, Nene P, Kachnic LA, Subramaniam RM. FDG PET/CT in the management of colorectal and anal cancers. AJR Am J Roentgenol 2014;203:1109-19. [Crossref] [PubMed]
- Ince S, Okuyucu K, Hancerliogulları O, Alagoz E, San H, Arslan N. Clinical Significance of Fluorine-18-fluorodeoxyglucose Positron Emission Tomography/computed Tomography in the Follow-up of Colorectal Cancer: Searching off Approaches Increasing Specificity for Detection of Recurrence. Radiol Oncol 2017;51:378-85. [Crossref] [PubMed]
- Kantorová I, Lipská L, Bêlohlávek O, Visokai V, Trubaĉ M, Schneiderová M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med 2003;44:1784-8.
- Zheng D, Niu L, Liu W, Zheng C, Yan R, Gong L, Dong Z, Li K, Fei J. Correlation analysis between the SUVmax of FDG-PET/CT and clinicopathological characteristics in oral squamous cell carcinoma. Dentomaxillofac Radiol 2019;48:20180416. [Crossref] [PubMed]
- Mantziari S, Pomoni A, Prior JO, Winiker M, Allemann P, Demartines N, Schäfer M. (18)F- FDG PET/CT-derived parameters predict clinical stage and prognosis of esophageal cancer. BMC Med Imaging 2020;20:7. [Crossref] [PubMed]
- Zhang H, Wroblewski K, Liao S, Kampalath R, Penney BC, Zhang Y, Pu Y. Prognostic value of metabolic tumor burden from (18)F-FDG PET in surgical patients with non-small-cell lung cancer. Acad Radiol 2013;20:32-40. [Crossref] [PubMed]
- Jiang H, Zhang R, Jiang H, Zhang M, Guo W, Zhang J, Zhou X, Pan W, Zhao S, Li P. Retrospective analysis of the prognostic value of PD-L1 expression and (18)F-FDG PET/CT metabolic parameters in colorectal cancer. J Cancer 2020;11:2864-73. [Crossref] [PubMed]
- Chen SW, Chiang HC, Chen WT, Hsieh TC, Yen KY, Chiang SF, Kao CH. Correlation between PET/CT parameters and KRAS expression in colorectal cancer. Clin Nucl Med 2014;39:685-9. [Crossref] [PubMed]
- Jo HJ, Kim SJ, Lee HY, Kim IJ. Prediction of survival and cancer recurrence using metabolic volumetric parameters measured by 18F-FDG PET/CT in patients with surgically resected rectal cancer. Clin Nucl Med 2014;39:493-7. [Crossref] [PubMed]
- Xu J, Li Y, Hu S, Lu L, Gao Z, Yuan H. The significant value of predicting prognosis in patients with colorectal cancer using (18)F-FDG PET metabolic parameters of primary tumors and hematological parameters. Ann Nucl Med 2019;33:32-8. [Crossref] [PubMed]
- Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Murakami K, Kitagawa Y. Metabolic Tumor Volume and Total Lesion Glycolysis in PET/CT Correlate With the Pathological Findings of Colorectal Cancer and Allow Its Accurate Staging. Clin Nucl Med 2016;41:761-5. [Crossref] [PubMed]
- Li C, Zhang D, Pang X, Pu H, Lei M, Fan B, Lv J, You D, Li Z, Zhang T. Trajectories of Perioperative Serum Tumor Markers and Colorectal Cancer Outcomes: A Retrospective, Multicenter Longitudinal Cohort Study. EBioMedicine 2021;74:103706. [Crossref] [PubMed]
- Zhu L, Ling C, Xu T, Zhang J, Zhang Y, Liu Y, Fang C, Yang L, Zhuang W, Wang R, Ping J, Wang M. Clinicopathological Features and Survival of Signet-Ring Cell Carcinoma and Mucinous Adenocarcinoma of Right Colon, Left Colon, and Rectum. Pathol Oncol Res 2021;27:1609800. [Crossref] [PubMed]
- Mylonas CC, Lazaris AC. Colorectal cancer and basement membranes: clinicopathological correlations. Gastroenterol Res Pract 2014;2014:580159. [Crossref] [PubMed]
- Bertero L, Massa F, Metovic J, Zanetti R, Castellano I, Ricardi U, Papotti M, Cassoni P. Eighth Edition of the UICC Classification of Malignant Tumours: an overview of the changes in the pathological TNM classification criteria-What has changed and why? Virchows Arch 2018;472:519-31.
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:329-59. [Crossref] [PubMed]
- Veit P, Kühle C, Beyer T, Kuehl H, Herborn CU, Börsch G, Stergar H, Barkhausen J, Bockisch A, Antoch G. Whole body positron emission tomography/computed tomography (PET/CT) tumour staging with integrated PET/CT colonography: technical feasibility and first experiences in patients with colorectal cancer. Gut 2006;55:68-73. [Crossref] [PubMed]
- de Geus-Oei LF, Ruers TJ, Punt CJ, Leer JW, Corstens FH, Oyen WJ. FDG-PET in colorectal cancer. Cancer Imaging 2006;6:S71-81. [Crossref] [PubMed]
- Otto AM. Warburg effect(s)-a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab 2016;4:5. [Crossref] [PubMed]
- Arslan E, Aksoy T, Gürsu RU, Dursun N, Çakar E, Çermik TF. The Prognostic Value of (18)F-FDG PET/CT and KRAS Mutation in Colorectal Cancers. Mol Imaging Radionucl Ther 2020;29:17-24. [Crossref] [PubMed]
- Ogawa S, Itabashi M, Kondo C, Momose M, Sakai S, Kameoka S. Prognostic Value of Total Lesion Glycolysis Measured by 18F-FDG-PET/CT in Patients with Colorectal Cancer. Anticancer Res 2015;35:3495-500.
- Kido H, Kato S, Funahashi K, Shibuya K, Sasaki Y, Urita Y, Hori M, Mizumura S. The metabolic parameters based on volume in PET/CT are associated with clinicopathological N stage of colorectal cancer and can predict prognosis. EJNMMI Res 2021;11:87. [Crossref] [PubMed]
- Li D, Wang Y, Liu W, Chen Q, Cai L, Xing X, Gao S. The Correlation between (18)F-FDG PET/CT Imaging SUVmax of Preoperative Colon Cancer Primary Lesions and Clinicopathological Factors. J Oncol 2021;2021:4312296. [Crossref] [PubMed]
- Zhang M, Yang J, Jiang H, Jiang H, Wang Z. Correlation between glucose metabolism parameters derived from FDG and tumor TNM stages and metastasis-associated proteins in colorectal carcinoma patients. BMC Cancer 2021;21:258. [Crossref] [PubMed]
- Yin YX, Xie MZ, Liang XQ, Ye ML, Li JL, Hu BL. Clinical Significance and Prognostic Value of the Maximum Standardized Uptake Value of (18)F-Flurodeoxyglucose Positron Emission Tomography-Computed Tomography in Colorectal Cancer. Front Oncol 2021;11:741612. [Crossref] [PubMed]
- O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420-5.
- Shi D, Cai G, Peng J, Li D, Li X, Xu Y, Cai S. The preoperative SUVmax for (18)F-FDG uptake predicts survival in patients with colorectal cancer. BMC Cancer 2015;15:991. [Crossref] [PubMed]
- Chen SH, Miles K, Taylor SA, Ganeshan B, Rodriquez M, Fraioli F, et al. FDG-PET/CT in colorectal cancer: potential for vascular-metabolic imaging to provide markers of prognosis. Eur J Nucl Med Mol Imaging 2021;49:371-84. [Crossref] [PubMed]


