
Direct invasion of mediastinal and vascular structures by aggressive inflammatory breast cancer: a rare case presentation
Introduction
Breast cancer ranks among the top causes of cancer-related deaths in women globally (1). The early detection of breast cancer has been demonstrated to reduce its burden and mortality. Consequently, numerous countries have implemented national breast cancer screening programs involving regular mammography for women. In many countries, women aged between 50 and 69 are targeted for screening due to their optimal benefit potential (2). Despite screening programs, breast cancer incidence and prognosis remain subjects of concern.
The two primary forms of invasive breast cancer differ in their origin within the breast tissue. Invasive ductal carcinoma (IDC) begins in milk ducts, making up about 80% of cases. Invasive lobular carcinoma (ILC), on the other hand, originates in lobules, constituting approximately 10% of diagnoses. In contrast to these, inflammatory breast cancer (IBC), though rare, is remarkably aggressive, representing roughly 2% of cases in the United States (3). It exhibits rapid progression and is characterized by symptoms resembling inflammation, such as redness, swelling, and warmth in the breast tissue. Breast cancer exhibits diverse molecular subtypes, including luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and triple-negative/basal-like. These subtypes have distinct molecular characteristics, prognoses, and responses to treatment.
Moreover, it’s important to note that the mediastinal invasion of breast cancer is a considerably rare occurrence. Hess et al. (4) reported that approximately 6% of breast cancers metastasize to mediastinal lymph nodes. Currently, there is no research on the direct invasion frequency of breast cancer into the mediastinum. This case report aims to emphasize the rare occurrence of mediastinal invasion in an advanced-stage luminal B subtype IBC, presented by a patient with symptoms resembling mastitis. Additionally, the report aims to provide a comprehensive overview of the patient’s clinical course.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
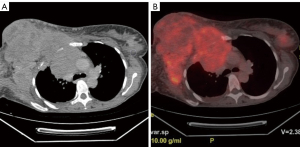
A 48-year-old female patient presented with complaints of swelling and pain in the breast and underarm region. This patient has no family history of breast cancer or any known risk factors. On physical examination, the right breast was found to be entirely firm and swollen, with palpable masses in the axillary region. An ultrasound examination revealed prominent skin and subcutaneous edema in both breasts, accompanied by heterogeneous hypervascular solid lesions filling the right breast and merging with each other. In order to establish a preliminary diagnosis of IBC and to evaluate the other breast, a breast magnetic resonance imaging (MRI) was performed. However, due to the right breast’s inability to fit into the coil, the scan could not be completed. To assess the extent of the condition, a thoracic computed tomography (CT) was performed, which showed that the lesion in the right breast was extending into the anterior mediastinum, causing destruction of the bone structures (Figure 1).
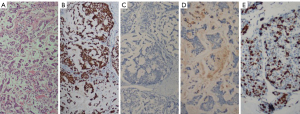
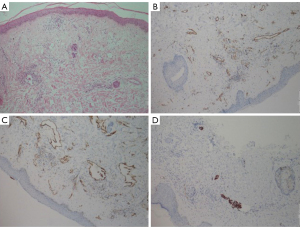
An excisional biopsy was performed on the skin and the mass. The pathology report revealed breast cancer with the following characteristics: estrogen receptor (ER): 100% (+++), progesterone receptor (PR): (−), C-erbB2: (−), ki67: 50%, p63: (−), GATA-binding protein 3 (GATA3): (+), epithelial membrane antigen (EMA): (+). The tumor was grade 3 (poorly differentiated). Furthermore, in the skin biopsy, tumor thrombi within dermal lymphatics were detected and showed positivity for cytokeratin 7 (CK7) (Figures 2,3).
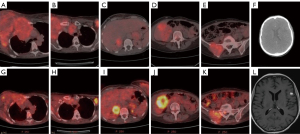
In positron emission tomography-CT (PET-CT) imaging, aside from the mediastinal invasion by the mass in the right breast, a mass in the left breast, as well as metastases in the lung, liver, brain, bilateral adrenal glands, bilateral axillary regions, and pelvic bones, were observed (Figure 4). Neoadjuvant chemotherapy (NAC) (carboplatin + paclitaxel protocol) and urgent palliative radiation therapy (RT) were initiated due to the advanced stage of the patient: trastuzumab-like targeted therapy could not be administered due to C-erbB2 negativity. Symptomatic treatment was commenced, including a proton pump inhibitor, corticosteroids, and diuretics, due to brain metastasis. A total dose of 30 Gray (Gy) of RT was administered in 10 fractions (fx) using a three-dimensional conformal technique, targeting the chest wall. Following 14 cycles of chemotherapy in combination with RT (30 Gy/10 fx), a subsequent PET CT scan revealed a regression in breast lesions and mediastinal invasion (attributed to RT). However, after systemic chemotherapy, there was observed progression in liver lesions (Figure 4). In conclusion, despite the regression in areas treated with RT, systemic chemotherapy proved ineffective in managing this aggressive case of breast cancer. The patient continues to receive palliative RT, targeting the cranial and lumbar regions (20 Gy/4 fx).
Discussion
IBC is a rare subtype among invasive breast cancers; however, it is characterized by an advanced stage at the time of diagnosis, aggressive progression, and an unfavorable prognosis (3).
The mediastinal invasion of breast cancer is a considerably rare occurrence. A conducted study reported a metastasis rate of 6% to mediastinal lymph nodes (4). However, the study did not provide specific details regarding the distinction between direct and indirect invasions. In our case, direct invasion into the mediastinum was observed. While there is currently no study demonstrating a direct correlation between mediastinal invasion and prognosis, it is worth noting that existing literature does not establish a clear relationship. In our case, mediastinal invasion regressed with neoadjuvant therapy. However, other organ metastases did not show regression, and there was evident progression in liver metastasis. The challenges associated with the treatment of mediastinal invasion, along with the increased risk of spreading to lymph nodes and neighboring vital organs, may be indicative of a poor prognosis. In cases of mediastinal invasion, RT should be incorporated into neoadjuvant therapy, and treatment should commence earlier with the goal of halting disease progression.
Nevertheless, it is important to highlight that the American Cancer Society (ACS) indicates a 5-year survival rate of 28% for individuals diagnosed with stage IV breast cancer. This percentage notably contrasts with the survival rates of earlier stages (5). Stage IV cancer typically has a poor prognosis, varying by cancer type. Treatment aims to improve quality of life, manage symptoms, and slow progression, not cure the disease.
Criteria for aortic invasion by thoracic masses on CT and conventional MRI included: obliteration of mediastinal fat between the mass and aorta, irregular aortic wall indentations by the mass, evaluation of mass-aorta contact extent (≥90° or ≥3 cm on axial images), and identification of an obtuse angle between mass and aorta (6). According to these criteria, suspicious aortic invasion was reported at points where mediastinal fat was obliterated (Figure 1).
Approximately 1/3 of IBC cases are stage IV at diagnosis. Metastatic cases undergo primary systemic therapy for optimal response. Those with significant clinical improvement post-therapy should receive intensive locoregional therapy. Surgery and radiation’s impact on overall survival in this context is disputed. Systemic treatment aims to extend survival, alleviate symptoms, and improve quality of life, despite associated toxicities.
The response to NAC varies based on different molecular subtypes in the treatment of breast cancer. Luminal B subtype, in particular, demonstrates a worse prognosis compared to luminal A, and the pathological complete response rates of luminal B are lower than those observed in the HER2-enriched and triple-negative subtypes (7). The addition of RT to neoadjuvant treatment, as in our case, enhances treatment efficacy. In our case, we observed a partial response to neoadjuvant treatment. According to the literature, luminal B tumors, while not as aggressive as triple-negative tumors, still exhibit a lower response rate compared to other subtypes.
In our case, progression was observed in liver metastases under systemic chemotherapy. The molecular subtype of metastatic lesions may differ from the primary lesion; therefore, it is necessary to plan a biopsy for the progressing liver metastasis.
In cases presenting with symptoms of mastitis, consideration should be given to inflammatory malignancies such as IBC, along with infectious and non-infectious mastitis, as part of the differential diagnosis.
In conclusion, this case report highlights the rare occurrence of mediastinal invasion in advanced luminal B subtype IBC. While the direct mediastinal invasion’s prognostic implications remain unclear, the aggressive nature of this subtype and its challenges in treatment are evident. Clinicians should consider such possibilities in mastitis-like symptoms. Tailored treatments based on molecular subtypes and careful monitoring are crucial. Further research is needed to better understand the significance of mediastinal invasion and improve treatment strategies for aggressive breast cancers.
Acknowledgments
I would like to extend my gratitude to Dr. Demet Kocatepe Çavdar from Bozyaka (Department of Pathology, Education and Research Hospital) for providing the microscopic images for this case report. These images have not been previously published elsewhere or subject to copyright protection.
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1220/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, Straif KInternational Agency for Research on Cancer Handbook Working Group. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med. 2015;372:2353-8. [Crossref] [PubMed]
- Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 2005;97:966-75. [Crossref] [PubMed]
- Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer 2006;106:1624-33. [Crossref] [PubMed]
- Survival Rates for Breast Cancer [Internet]. Cancer.org. 2018 [cited 2023 Aug 26]. Available online: https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
- Hong YJ, Hur J, Lee HJ, Kim YJ, Hong SR, Suh YJ, Choi BW. Respiratory dynamic magnetic resonance imaging for determining aortic invasion of thoracic neoplasms. J Thorac Cardiovasc Surg 2014;148:644-50. [Crossref] [PubMed]
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [Crossref] [PubMed]


