
A retrospective study of 3D measurement and analysis applied in the morphological evaluation of achalasia cardia
Introduction
Achalasia cardia (AC) is defined as a rare esophageal smooth muscle disorder characterized by impaired relaxation of the lower esophageal sphincter (LES) and absent or spastic esophageal body contractions (1). Its typical symptoms include dysphagia, regurgitation, chest pain, and weight loss (2). AC can be categorized into three subtypes on the basis of its diagnostic gold standard test, namely, high-resolution manometry (HRM) (2,3). However, given the invasiveness of HRM, a good number of patients cannot tolerate this test, which may lead to missed diagnosis, misdiagnosis, and delayed diagnosis and treatment of AC. In light of this condition, researchers are increasingly inclined to develop new, reliable, and noninvasive means for the diagnosis and treatment of AC. Some studies have been conducted in 2D planes. Licurse et al. proved the effectiveness of chest computed tomography (CT) in terms of differentiating primary and secondary AC (4). Ishii et al. found that performing chest CT scan in a timely fashion can avoid the delayed diagnosis of AC (5). CT esophagram was also found to be useful for the evaluation of post-peroral endoscopic myotomy (POEM) management (6). Through 2D measurement, researchers can observe esophageal thickness and esophageal length, but they cannot measure the angle relationship between the esophagus and its adjacent anatomical structure, which may be closely associated with clinical symptoms and prognosis of AC and can be measured accurately by 3D reconstruction.
The 3D reconstruction technique based on CT imaging has been increasingly used in some areas of diseases, such as female pelvic tumors, prostatic hyperplasia, and infertility, especially as an auxiliary method for some surgical operations (7-11). A prospective study investigated differences in the 3D pressure profile of LES and hiatal contraction between normal subjects and patients with AC, their results indicated the anatomical and functional abnormalities of the crural diaphragm muscle in patients with AC, suggesting that creative 3D reconstruction can be regarded as a promising direction for AC management (12).
Collectively, the objectives of our study were as follows. First, we aimed to carry out 3D reconstruction based on CT imaging to observe anatomical features of healthy subjects and patients with AC. Second, we planned to perform 3D measurement according to the reconstructed anatomical structure including esophagus zone, stomach zone, spine, left crus, and right crus. Finally, we would compare the differences in parameters on the basis of various grouping methods. This work was designed to contribute to the wide application of 3D measurement and further development of an alternative non-invasive diagnostic approach for AC. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-626/rc).
Methods
Study population and data collection
This cross-sectional retrospective study was conducted between January 2018 and October 2022. It included 160 patients diagnosed with AC by HRM from Tianjin Medical University General Hospital. The exclusion criteria were as follows: patients combined with other esophageal-related diseases, patients combined with other chronic diseases or malignant tumors, or patients without complete CT image data. All of the clinical and imaging materials were collected and assessed by two researchers. Through this process, 126 patients were included for final analysis (Figure 1). The clinical information and CT image data from 40 healthy test subjects were also collected in this research as controls. These healthy individuals were shown to be free from any esophageal motility disorders, chronic diseases, or malignant tumors. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Medical University General Hospital (No. IRB2023-WZ-054) and individual consent for this retrospective analysis was waived.

3D reconstruction
The axial 2D CT images in DICOM format with a slice thickness of 5 mm were imported into Mimics software 19.0 (Materialize, Leuven, Belgium). Subsequently, we reconstructed the model of the esophagus zone, stomach zone, spine, left crus, and right crus and used Geomagic Studio 14.0 (Geomagic, Rock Hill, SC, USA) for further smoothing. Finally, we used 3-matic software 11.0 (Materialize, Leuven, Belgium) to carry out measurements and analyses on the basis of the reconstructed 3D models. All 3D reconstructions, measurements, and analyses were completed and checked by three researchers.
Parameter measurements and analysis
Through 3-matic software, we measured the thoracic esophagus length, retrocardiac esophagus length, and intra-abdominal LES length by generating the esophagus centerline. Esophagus maximum transverse diameter and maximum longitudinal diameter were defined as the length of the line from the leftmost point to the rightmost point and the length of the line from the frontmost point to the lastmost point in 3D models, respectively. The volume of the esophagus can be viewed and calculated by “Mimics software → properties”. We also measured the max thickness of the esophageal wall.
Standard sagittal and coronal planes were made through the center of the tenth thoracic cone. In the sagittal plane, the thoracic esophagus-spine angle was defined as the angle between the sagittal plane and thoracic esophagus centerline, whereas the retrocardiac esophagus-spine angle was defined as the angle between the sagittal plane and retrocardiac esophagus centerline. The spine-LES angle was defined as the angle between the intra-abdominal LES and the plane (sagittal and coronal). We also defined right deflection angle as the positive angle and left deflection angle as the negative angle.
The gastroesophageal insertion angle (His angle) was defined as the smallest angle, which was formed by the intra-abdominal LES centerline and gastroesophageal line.
Statistical analysis
Characteristics of participants were described. Normally distributed continuous variables were presented as mean and standard deviation (SD), whereas non-normally distributed continuous variables were presented as median and interquartile range (IQR). Categorical variables were presented as number and percentage. Two independent-sample t-tests or Mann-Whitney U test was used to compare the measurement parameters of different groups. The Shapiro-Wilk test was used to conduct the normality test with sample size less than 50. The Kolmogorov-Smirnov test was used to conduct the normality test with sample size more than 50. Comparisons between different groups were made by one-way analysis of variance (ANOVA) or Kruskal-Wallis test. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 24 (IBM, Armonk, NY, USA).
Results
Among 126 patients with AC, 45 patients were classified as type I, 66 were classified as type II, and 15 were classified as type III. Our cohort contained 56 men and 70 women, with a median age of 51 [36–62.50], 47 [34–61], and 48 [31–63] years. Figure 2 shows the 3D models of the esophagus zone, stomach zone, spine, left crus, and right crus and compared measurements between healthy subjects and different AC subtypes. In Figures 3-5, we depicted one typical model of a patient with AC to demonstrate some essential parameters visually and quantitatively, including esophagus length, esophagus maximum transverse and longitudinal diameter, volume of esophagus, esophageal thickness, His angle, and spine-LES angle.



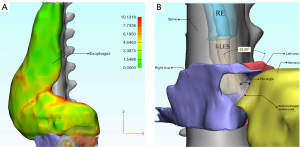
Table 1 presents a comparison between healthy controls and patients with AC. Retrocardiac esophagus length, volume of esophagus, max thickness of esophageal wall, esophagus maximum transverse diameter, esophagus maximum longitudinal diameter, thoracic esophagus-spine angle, and spine-LES angle all presented statistical differences.
Table 1
| Parameters | Control (n=40) | AC (n=126) | P |
|---|---|---|---|
| Age (years) | 52.50 (46.25, 57.75) | 48 (34, 61.25) | 0.694 |
| Sex | 0.643 | ||
| Male | 20 (50.00) | 56 (44.40) | |
| Female | 20 (50.00) | 70 (55.60) | |
| Measurement indicators | |||
| TEL (cm) | 8.84 (8.39, 9.75) | 9.23 (8.15, 10.70) | 0.342 |
| REL (cm) | 8.64 (8.13, 9.52) | 9.84 (8.63, 11.07) | 0.006* |
| I-LESL (cm) | 4.63 (3.38, 5.35) | 4.31 (3.27, 5.61) | 0.785 |
| VE (mm3) | 30,156.50 (26,987.50, 33,765) |
137,021 (61,866.78, 234,463.33) |
<0.001* |
| His angle (°) | 81.84 (74.57, 97.28) | 88.26 (74.53, 102.46) | 0.183 |
| Max thickness of EW (mm) | 4.10 (3.55, 4.60) | 7.18 (6.06, 8.97) | <0.001* |
| EMTD (cm) | 1.91 (1.60, 2.21) | 3.83 (2.65, 5.19) | <0.001 |
| EMLD (cm) | 2.02 (1.71, 2.29) | 2.72 (1.89, 3.55) | 0.006* |
| TESA (°) | −5.18 (−7.43, −3.10) | 8.55 (2.80, 14.18) | <0.001* |
| RESA (°) | 4.33 (−2.55, 5.99) | 3.86 (−7.46, 13.06) | 0.509 |
| Spine-LES angle in sagittal plane (°) | −19.09 (−23.94, −12.84) | −24.96 (−34.19, −14.30) | 0.028* |
| Spine-LES angle in coronal plane (°) | 164.42 (157.88, 168.99) | 170.70 (161.33, 175.15) | 0.045* |
Data are presented as median (IQR) or n (%). *, P<0.05. AC, achalasia cardia; TEL, thoracic esophagus length; REL, retrocardiac esophagus length; I-LESL, intra-abdominal lower esophageal sphincter length; VE, volume of esophagus; His angle, gastroesophageal insertion angle; EW, esophageal wall; EMTD, esophagus maximum transverse diameter; EMLD, esophagus maximum longitudinal diameter; TESA, thoracic esophagus-spine angle; RESA, retrocardiac esophagus-spine angle; LES, lower esophageal sphincter; IQR, interquartile range.
Table 2 shows the characteristics of patients according to manometric types. HRM parameters, thoracic esophagus length, intra-abdominal LES length, volume of esophagus, His angle, esophagus maximum transverse diameter, esophagus maximum longitudinal diameter, and thoracic esophagus-spine angle all presented statistical differences. Figure 6 shows that manometric types were positively associated with His angle [r=0.196; 95% confidence interval (CI): 0.009, 0.372; P=0.028] but negatively associated with volume of esophagus (r=−0.480; 95% CI: −0.639, −0.310; P<0.001), esophagus maximum transverse diameter (r=−0.551; 95% CI: −0.679, −0.400; P<0.001), esophagus maximum longitudinal diameter (r=−0.518; 95% CI: −0.649, −0.366; P<0.001), and thoracic esophagus-spine angle (r=−0.324; 95% CI: −0.479, −0.157; P<0.001).
Table 2
| Parameters | Type I (n=45) | Type II (n=66) | Type III (n=15) | P |
|---|---|---|---|---|
| Age (years) | 51 [36, 62.50] | 47 [34, 61] | 48 [31, 63] | 0.735 |
| Sex | 0.225 | |||
| Male | 22 (48.90) | 25 (37.90) | 9 (60.00) | |
| Female | 23 (51.10) | 41 (62.10) | 6 (40.00) | |
| Measurement indicators | ||||
| DCI (mmHg·s·cm) | 12.90 [6.30, 38.80] | 75.79 [49.18, 90.98] | 1,136.20 [862, 2,005] | <0.001* |
| LES IRP 4 s (mmHg) | 20.20 [16.55, 24.40] | 34.40 [25.03, 47.20] | 28.70 [23.50, 35.70] | <0.001* |
| DL (s) | 10.80 [8.10, 14.12] | 7.95 [6.22, 11.25] | 4.42 [3.57, 5.52] | 0.001* |
| TEL (cm) | 10.35 [8.76, 10.99] | 8.73 [7.59, 10.02] | 9.60 [8.78, 11.25] | 0.005* |
| REL (cm) | 10.06±2.35 | 9.88±1.94 | 9.53±1.57 | 0.681 |
| I-LESL (cm) | 4.77 [3.67, 6.13] | 3.85 [2.73, 5.16] | 4.95 [3.63, 5.91] | 0.004* |
| VE (mm3) | 233,015 [181,310.50, 357,935] |
83,834.22 [43,201.69, 158,681.92] |
118,320 [23,805, 281,898] |
<0.001* |
| His angle (°) | 76.47±25.19 | 96.78±15.76 | 78.09±20.22 | <0.001* |
| Max thickness of EW (mm) | 8.13 [6.23, 9.98] | 6.76 [5.81, 7.96] | 7.38 [6.63, 8.46] | 0.075 |
| EMTD (cm) | 5.32 [4.06, 6.31] | 3.09 [1.99, 4] | 3.49 [1.55, 4.62] | <0.001* |
| EMLD (cm) | 3.71 [2.79, 5.10] | 2.16 [1.60, 2.91] | 2.70 [1.30, 3.24] | <0.001* |
| TESA (°) | 12.94 [6.36, 16.21] | 7.77 [2.02, 12.31] | 2.27 [0.52, 8.83] | 0.001* |
| RESA (°) | 2.69 [−10.71, 12.13] | 5.30 [−5.31, 14.68] | 2.85 [−11.54, 11.07] | 0.188 |
| Spine-LES angle in sagittal plane (°) | −22.69 [−41.57, −10.95] | −26.76 [−33.44, −15.75] | −25.15 [−27.29, −19.41] | 0.543 |
| Spine-LES angle in coronal plane (°) | 170.42 [158.78, 175.23] | 170.74 [161.78, 175.83] | 169.54 [163.94, 173.84] | 0.916 |
Data are presented as median [IQR], n (%), or mean ± SD. *, P<0.05. DCI, distal contractile integral; LES IRP 4 s, the 4 s integrated relaxation pressure of lower esophageal sphincter; DL, distal latency; TEL, thoracic esophagus length; REL, retrocardiac esophagus length; I-LESL, intra-abdominal lower esophageal sphincter length; VE, volume of esophagus; His angle, gastroesophageal insertion angle; EW, esophageal wall; EMTD, esophagus maximum transverse diameter; EMLD, esophagus maximum longitudinal diameter; TESA, thoracic esophagus-spine angle; RESA, retrocardiac esophagus-spine angle; LES, lower esophageal sphincter; IQR, interquartile range; SD, standard deviation.

Discussion
In this retrospective study, we conducted 3D reconstruction and measurements based on CT imaging and identified some essential parameters, which can be used to differentiate healthy controls and three subtypes of AC. The results of our study demonstrated the feasibility of 3D reconstruction and measurement for the management of AC. First, we found that some parameters present statistical differences between patients with AC and healthy controls, including retrocardiac esophagus length, volume of esophagus, max thickness of esophageal wall, esophagus maximum transverse diameter, esophagus maximum longitudinal diameter, thoracic esophagus-spine angle, and spine-LES angle (Table 1). Although HRM is a golden standard for AC diagnosis, its invasiveness makes it unacceptable and unfriendly to some patients in clinical practice. Moreover, some patients suffering severe dysphagia have difficulties in receiving the contrast medium for the examination of esophagography. Considering the above facts, we believe that 3D reconstruction can be used as a more convenient screening tool for the assessment of esophageal structure and function by measuring the parameters acquired from the safe and harmless CT scan. Second, some parameters can also be used to differentiate three subtypes of AC statistically, such as esophageal length, volume of esophagus, His angle, esophagus maximum transverse and longitudinal diameter, and thoracic esophagus-spine angle (Table 2). Further, manometric types were correlated with His angle, esophageal volume, thoracic esophagus-spine angle, esophagus maximum transverse diameter, and esophagus maximum longitudinal diameter (Figure 6). These all indicated that 3D parameters could be potentially regarded as AC-specific parameters. For patients who are unwilling and unable to receive HRM, 3D reconstruction could be an effective and safe alternative to evaluate their condition preliminarily.
In detail, the parameters of our 3D models could be divided into three groups. One group was used to assess the dilation grading and morphological change of the esophagus and the corresponding parameters including esophageal thickness and volume of esophagus. Our results showed that the above parameters of patients with AC were higher than those of healthy subjects, which were consistent with the fact that patients with AC were more likely to have esophageal dilation than healthy subjects. Among three subtypes, type I AC patients tended to have larger volume of esophagus and esophageal thickness, which was in line with the study that this type of patients tended to have severer dilation (13). The other group was used to evaluate the symptoms of AC. We selected esophageal length and His angle to assess the degree of reflux, which have been studied in gastroesophageal reflux disease (GERD) (14,15). Studies have reported that patients with shorter intra-abdominal LES length and larger His angle have severer reflux than their counterparts in GERD (16,17). In our research, patients with type II AC also had shorter intra-abdominal LES length and larger His angle, indicating that this type of patients appeared to have severer reflux symptom than the two other subtypes. The luminal change has shown to be related to the severity of AC in 2D plane, so we similarly measured esophagus maximum transverse and longitudinal diameter in 3D plane (18). In our study, patients with type I AC tended to have larger esophagus maximum transverse diameter and longitudinal diameter. This can be explained by the fact that type I AC was more intended to develop to the end-stage of AC and thus had the severer condition (1). Another group was used to assess the esophageal tortuosity and corresponding parameters including esophagus-spine angle and spine-LES angle. We found no relevant studies on esophageal tortuosity in 3D models. Previous studies mostly centered on the changes in esophageal tortuosity in the 2D plane and have found that POEM can ameliorate reflux symptoms by increasing the angle of esophageal tortuosity (19,20). Thus, we believe it is meaningful to measure esophagus-spine angle in 3D plane. But in our research, we cannot reach a solid conclusion that esophagus-spine angle was associated with the reflux symptom. The spine-LES angle was used to assess the pressure of LES (12). Our results showed that the spine-LES angle in AC group was larger than that of healthy control, which was contrary to previous study. We believe these issues can be addressed by expanding sample size and certainly worthy of investigating further. Through 3D reconstruction, we assessed the dilation grading and esophageal morphology and measured some essential parameters, to evaluate the anatomic abnormality of the esophagus, which may be associated with clinical symptoms and predict prognosis. We believe this advantage can become a promising direction in AC and even other esophageal motility disorders, as well as benefit patients.
This study had some limitations. First, although we reconstructed the 3D model of the esophagus, stomach, spine, and crus and provided a new method for the management of AC, this method must still be performed manually, so it is time-consuming and causes selection bias. We should develop an automatic means to complete these complicated procedures in the future. Second, we only included 40 healthy subjects, which may cause data bias. Third, although AC is a rare digestive disease, our sample size must be expanded for further study. Finally, our study was only a retrospective study on healthy controls and patients with AC, without the analysis and comparison of other esophageal motility diseases. This step will be performed in our further research. Taken together, this creative method can be potentially regarded as a noninvasive and precise alternative for the management of AC.
Conclusions
This study successfully presented the differences in 3D parameters between healthy subjects and different AC subtypes. The 3D reconstruction and measurement could be regarded as a good support for developing non-invasive tools for AC management.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-626/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-626/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Medical University General Hospital (No. IRB2023-WZ-054) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Savarino E, Bhatia S, Roman S, Sifrim D, Tack J, Thompson SK, Gyawali CP. Achalasia. Nat Rev Dis Primers 2022;8:28. [Crossref] [PubMed]
- Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am J Gastroenterol 2020;115:1393-411. [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 2008;135:1526-33. [Crossref] [PubMed]
- Licurse MY, Levine MS, Torigian DA, Barbosa EM Jr. Utility of chest CT for differentiating primary and secondary achalasia. Clin Radiol 2014;69:1019-26. [Crossref] [PubMed]
- Ishii T, Akaishi T, Abe M, Takayama S, Koseki K, Kamei T, Nakano T. Importance of Barium Swallow Test and Chest CT Scan for Correct Diagnosis of Achalasia in the Primary Care Setting. Tohoku J Exp Med 2019;247:41-9. [Crossref] [PubMed]
- Pannu D, Yang D, Abbitt PL, Draganov PV. Prospective evaluation of CT esophagram findings after peroral endoscopic myotomy. Gastrointest Endosc 2016;84:408-15. [Crossref] [PubMed]
- Liu Y, Meng S, Wei M, Dai F, Xiao L, Mu X, Tang J. Reconstruction of three-dimensional models for complex female pelvic tumors. Int J Gynaecol Obstet 2022;157:747-9. [Crossref] [PubMed]
- Watanabe M, Murakami R, Miyauchi R, Amano N, Moriuchi Y, Imachi K. Utility of Preoperative Multidetector-Row Computed Tomographic Angiography after Sublingual Nitroglycerin with Three-Dimensional Reconstruction in Planning of the Anterolateral Thigh Flap. Plast Reconstr Surg 2020;145:407e-11e. [Crossref] [PubMed]
- Feng Y, Wu J, Zhu H, Wang Q, Li T, Xu Y, Zhang P, Zhai L. Three-dimensional measurement and analysis of benign prostatic hyperplasia. Transl Androl Urol 2021;10:2384-96. [Crossref] [PubMed]
- Feng Y, Zhang S, Zhou Y, He G, Hong L, Shi L, Wang J, Zhang P, Zhai L. Three-dimensional measurement and analysis of morphological parameters of the uterus in infertile women. Quant Imaging Med Surg 2022;12:2224-37. [Crossref] [PubMed]
- Chin W, Zhang R, Zhang Q, Xu Z, Li D, Wu J. Modifications of three-dimensional costal cartilage framework grafting in auricular reconstruction for microtia. Plast Reconstr Surg 2009;124:1940-6. [Crossref] [PubMed]
- Mittal RK, Kumar D, Kligerman SJ, Zifan A. Three-Dimensional Pressure Profile of the Lower Esophageal Sphincter and Crural Diaphragm in Patients with Achalasia Esophagus. Gastroenterology 2020;159:864-872.e1. [Crossref] [PubMed]
- Sato H, Fujiyoshi Y, Abe H, Shiwaku H, Shiota J, Sato C, Sakae H, Ominami M, Hata Y, Fukuda H, Ogawa R, Nakamura J, Tatsuta T, Ikebuchi Y, Yokomichi H, Terai S, Inoue H. Development of Dilated Esophagus, Sigmoid Esophagus, and Esophageal Diverticulum in Patients With Achalasia: Japan Achalasia Multicenter Study. J Neurogastroenterol Motil 2022;28:222-30. [Crossref] [PubMed]
- Koumanidou C, Vakaki M, Pitsoulakis G, Anagnostara A, Mirilas P. Sonographic measurement of the abdominal esophagus length in infancy: a diagnostic tool for gastroesophageal reflux. AJR Am J Roentgenol 2004;183:801-7. [Crossref] [PubMed]
- Zhang R, Li Z, Li C, Ji F, Han X, Wang Z. Effect of laparoscopic angle of His reconstruction in the treatment of patients with gastroesophageal reflux disease and hiatal hernia. Chin Med J (Engl) 2022;135:1750-2. [Crossref] [PubMed]
- Fujiwara Y, Nakagawa K, Kusunoki M, Tanaka T, Yamamura T, Utsunomiya J. Gastroesophageal reflux after distal gastrectomy: possible significance of the angle of His. Am J Gastroenterol 1998;93:11-5. [Crossref] [PubMed]
- Bochkarev V, Lee YK, Vitamvas M, Oleynikov D. Short esophagus: how much length can we get? Surg Endosc 2008;22:2123-7. [Crossref] [PubMed]
- Csendes P, Csendes A, Cortes C, Burgos AM. Evolutive radiological changes of the esophagus in patients with achalasia who did not receive treatment. Surg Today 2007;37:183-6. [Crossref] [PubMed]
- Yoon HJ, Lee JE, Jung DH, Park JC, Youn YH, Park H. Morphologic Restoration After Peroral Endoscopic Myotomy in Sigmoid-type Achalasia. J Neurogastroenterol Motil 2020;26:67-73. [Crossref] [PubMed]
- Xu J, Zhong C, Huang S, Zeng X, Tan S, Shi L, Peng Y, Lü M, Ma L, Tang X. Efficacy and Safety of Peroral Endoscopic Myotomy for Sigmoid-Type Achalasia: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2021;8:677694. [Crossref] [PubMed]


