
Giant ruptured abdominal aortic aneurysm: a case description
Introduction
An abdominal aortic aneurysm (AAA) is defined as a dilation of the infrarenal aorta to a diameter greater than 30 mm. At present, the indication for repair is related to the maximal diameter of the abdominal aorta measured in computed tomography angiography (CTA) or/and using an ultrasound scan; the two most used imaging modalities for monitoring aneurysm progression. CTA is essential in the diagnosis of acute aortic syndromes. Elective endovascular or open AAA repair for asymptomatic patients is recommended when a maximum diameter of 55 mm is reached or an expansion rate of 10 mm/year is observed (1). The rupture risk for small AAAs is higher in women than in men which affects the recommended maximal diameter threshold for therapy depending on the sex (55 mm in men and ≥50 mm in women if operative risk is reasonable). Moreover, the presence of abdominal pain, back pain or limb ischemia increases the risk for AAA rupture, and thus, patients with symptomatic AAA should undergo repair procedures. Aneurysm size is the most associated to the likelihood of rupture and the risk is significantly higher in large aneurysms. Literature describing giant AAAs, defined as ≥130 mm in maximal diameter is scarce (2). Such large-sized AAAs, as the one presented in our case study are very rare. Besides the diameter size, other parameters such as advancing age, male gender, hypertension, smoking, peak wall stress, and intraluminal thrombus (ILT) thickness have also been found to predispose to AAA rupture (3).
According to the 2019 ESVS Clinical practice guidelines, AAA prevalence (in European countries) is reported 1.3–3.3% among men 65 years or older and is higher than 5% in males with a smoking history according to the US data (4). The prevalence is lower in women.
Case presentation
A 79-year-old woman complaining of a strong pain in the left side of the abdomen was admitted to the Department of Vascular Surgery and Angiology of Pomeranian Medical University. A pulsatile, expansile abdominal mass was suggestive of an AAA. Further physical examination revealed a heart rate of 90 beats/min and blood pressure of 105/70 mmHg. The femoral pulses were palpable on both sides. The patient’s medical history included smoking, and well-controlled hypertension and diabetes. She had not sought care and had undergone no previous workup for the AAA. Laboratory findings were: hemoglobin 7.8 mmol/L (reference range, 7.7–10 mmol/L), red blood cell count 4.06×106/µL (reference range, 4.0×106–5.0×106/µL), hematocrit 36.5% (reference range, 37–47%), white blood cell count 18.75×103/µL (reference range, 4.0×103–10.0×103/µL), C-reactive protein 2.2 mg/dL (reference range, 0–5 mg/dL), neutrophil to lymphocyte ratio (NLR) 5.2 (reference range, 0.78–3.53), and D-dimers 7,650 ng/mL (reference range, 0–500 ng/mL).
An emergency CTA revealed a large ruptured fusiform AAA measuring 158 mm × 141 mm × 170 mm and a retroperitoneal hematoma (Figure 1). Multiplanar reconstruction of the computed tomography of the AAA showed a sagittal image obtained with intravenous contrast material in the late arterial phase with extravasation of contrast material—a finding suggestive of an active bleeding from the posterolateral ruptured aneurysm (Figure 1B, arrow). The aneurysm morphology was evaluated for eligibility for the endovascular repair (EVAR) technique. The aneurysm neck length was 8 mm and there was severe angulation (>75 degree) between aneurysm neck and the aneurysm. The AAA extended to the left common iliac artery reaching the maximal diameter of 45 mm. Considering the anatomical conditions the patients was ineligible for the endovascular approach.
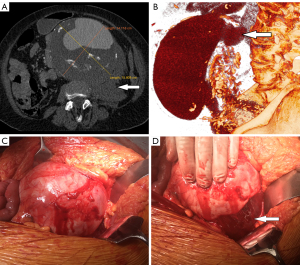
The patient was immediately taken to the operating theater. In many cases open surgical repair is the only available treatment for giant AAAs. Unlike endovascular therapy of large AAA which requires anatomical criteria to be met, open surgery can be performed regardless of anatomical constraints. She underwent surgery, during which the aneurysm and retroperitoneal hematoma were exposed (Figure 1C,1D) and replaced with an aortobifemoral bypass graft. The aneurysm was clamped below the renal arteries for more than 30 minutes. The proximal anastomosis was performed end-to-end while both distal were end-to-side to the common femoral arteries. The proximal anastomosis was performed using the parachute sewing technique. After the aneurysm repair was completed, the patient died soon after the wounds were closed on the operating table.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for all procedures performed in this study. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
AAA is often identified at various stages as a result of incidental imaging. Most frequently the initial physical findings in misdiagnosed AAA ruptured patients involved abdominal pain (70%), shock (57%), and back pain (50%) (5). Rupture of an AAA is associated with a high risk of death with a mortality of 85–90%. Increasing maximal diameter is one of the main risk factors for AAA rupture.
The amount and distribution of elastin-rich elastic fibers vary within tissues and organs. The maintenance of arterial elastin’s integrity is essential for the prevention of AAA development. We suggest that within aortic wall certain focal points may be found with genetically determined de novo reduced amounts of elastin and elastic fibers, which favor early rupture. On the other hand, a genetic predisposition to homogeneity and a high density net of elastic fibers within the aortic wall may play a protective role against rupture and favor giant aneurysm development. Also, ILT thickness is involved in the elastin degradation and vascular degeneration found in AAA. The amount of elastin fibers in the thin thrombus-covered wall was reduced when compared with the wall covered by thick ILT (3). Therefore, we recommend performing a histological examination of the AAA wall. Aortic metabolic activity is suggested to correlate with the presence and progression of AAA but does not correlate with aneurysm size (6). Moreover, some authors reported that larger aneurysms present factors allowing them to reach such sizes that make them more resistant to earlier rupture, as opposed to the ones that never reach prominent sizes. This theory is explained by regional differences, such as heterogeneity and localized pathologies, within the AAA wall (7).
Small variations in geometry may alter the flow field and the stresses exerted on the walls therefore affecting the rupture risk of the aneurysm. Moreover, peak wall rupture index is greater in ruptured than asymptomatic intact AAAs of similar maximum aortic diameter (8).
D-dimers are elevated in large AAAs, and in our patient D-dimers reached 7,650 ng/mL. Vele et al. reported a strong positive correlation between AAA progression and increasing plasma D-dimers over a 1-year period (9).
It has been reported that NLR is significantly higher among patients with ruptured AAA and that an NLR >5 (in this case was 5.2) indicates a five times greater possibility of aneurysm being ruptured (10). Raised NLR also demonstrates a significantly increased risk of mortality after AAA surgical repair (11). In our opinion, D-dimers and NLR might be useful, as additional parameters to ultrasound monitoring of small aneurysms and could be used to help identify rupture risk.
AAAs with a diameter over 50 mm ruptured more frequently in the posterolateral wall (67%) in a prospective autopsy study (12). Therefore, as presented in this case, most of them are associated with retroperitoneal hematoma. We observed that patients with intraperitoneal ruptures of the AAAs almost never make it to hospital.
Currently, most of the AAAs are treated with EVAR. Although EVAR has been proven to have lower periprocedural mortality than open surgical repair, giant AAAs due to the anatomic limitations, are still repaired by standard open surgery (1).
Conclusions
It is not yet conclusive why giant AAAs manage to reach extreme sizes without rupturing. To recapitulate, giant aneurysms pose a high risk, and are associated with treatment difficulties and severe complications.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-747/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for all procedures performed in this study. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, Augoustides JG, Beck AW, Bolen MA, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334-482. [Crossref] [PubMed]
- Ullery BW, Itoga NK, Lee JT. Giant Abdominal Aortic Aneurysms: A Case Series and Review of the Literature. Vasc Endovascular Surg 2015;49:242-6. [Crossref] [PubMed]
- Wiernicki I, Parafiniuk M, Kolasa-Wołosiuk A, Gutowska I, Kazimierczak A, Clark J, Baranowska-Bosiacka I, Szumilowicz P, Gutowski P. Relationship between aortic wall oxidative stress/proteolytic enzyme expression and intraluminal thrombus thickness indicates a novel pathomechanism in the progression of human abdominal aortic aneurysm. FASEB J 2019;33:885-95. [Crossref] [PubMed]
- Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019;57:8-93. [Crossref] [PubMed]
- Marston WA, Ahlquist R, Johnson G Jr, Meyer AA. Misdiagnosis of ruptured abdominal aortic aneurysms. J Vasc Surg 1992;16:17-22. [Crossref] [PubMed]
- Barwick TD, Lyons OT, Mikhaeel NG, Waltham M, O’Doherty MJ. 18F-FDG PET-CT uptake is a feature of both normal diameter and aneurysmal aortic wall and is not related to aneurysm size. Eur J Nucl Med Mol Imaging 2014;41:2310-8. [Crossref] [PubMed]
- Tavares Monteiro JA, da Silva ES, Raghavan ML, Puech-Leão P, de Lourdes Higuchi M, Otoch JP. Histologic, histochemical, and biomechanical properties of fragments isolated from the anterior wall of abdominal aortic aneurysms. J Vasc Surg 2014;59:1393-401.e1-2.
- Singh TP, Moxon JV, Gasser TC, Golledge J. Systematic Review and Meta-Analysis of Peak Wall Stress and Peak Wall Rupture Index in Ruptured and Asymptomatic Intact Abdominal Aortic Aneurysms. J Am Heart Assoc 2021;10:e019772. [Crossref] [PubMed]
- Vele E, Kurtcehajic A, Zerem E, Maskovic J, Alibegovic E, Hujdurovic A. Plasma D-dimer as a predictor of the progression of abdominal aortic aneurysm. J Thromb Haemost 2016;14:2298-303. [Crossref] [PubMed]
- Aurelian SV, Adrian M, Andercou O, Bruno S, Alexandru O, Catalin T, Dan B. Neutrophil-to-Lymphocyte Ratio: A Comparative Study of Rupture to Nonruptured Infrarenal Abdominal Aortic Aneurysm. Ann Vasc Surg 2019;58:270-5. [Crossref] [PubMed]
- Xu Y, Fang H, Qiu Z, Cheng X. Prognostic role of neutrophil-to-lymphocyte ratio in aortic disease: a meta-analysis of observational studies. J Cardiothorac Surg 2020;15:215. [Crossref] [PubMed]
- Simão da Silva E, Rodrigues AJ, Magalhães Castro de Tolosa E, Rodrigues CJ, Villas Boas do Prado G, Nakamoto JC. Morphology and diameter of infrarenal aortic aneurysms: a prospective autopsy study. Cardiovasc Surg 2000;8:526-32. [Crossref] [PubMed]


