
Predictive value of metabolic parameters derived from preoperative 18F-FDG positron emission tomography/computed tomography for brain metastases in patients with surgically resected non-small cell lung cancer
Introduction
Brain metastases (BMs) are common complications in patients with non-small cell lung cancer (NSCLC). Approximately 6.2–20.9% of patients with surgically resected NSCLC will develop BM during follow-up (1-3), which significantly impairs neurocognition, reduces quality of life, and increases mortality (4). The therapeutic management of BM has expanded from surgery and whole-brain radiotherapy to stereotactic radiosurgery, targeted therapies, and immunotherapies, which are often used in combination or sequentially (5). Early BM detection is crucial and will help conduct timely salvage therapies to improve neurological sequelae and survival. However, according to several guidelines, brain magnetic resonance imaging (MRI) is not routinely performed in asymptomatic patients with surgically resected NSCLC (6,7). Diagnosis of BM is frequently missed until a patient exhibits significant neurological symptoms. Therefore, identifying patients with NSCLC at a higher risk of developing BM may help implement individualized surveillance strategies.
BM develops due to hematogenous cells spreading from the primary tumor to the brain microvasculature. Cancerous cells must first separate from the primary tumor, infiltrate the surrounding tissues, and enter the vasculature and lymphatic system (8). Therefore, assessing tumor aggressiveness can help predict the occurrence of BM. For patients with surgically resected NSCLC, the Tumor Node Metastasis (TNM) staging system reflects the invasiveness and growth characteristics of the tumor (9). Several studies have found that NSCLC patients with higher T, N, and TNM stages are more likely to develop BM (10,11), while other studies do not show similar results (12,13). One probable explanation for this difference is that tumor-specific factors are various, creating a heterogeneous subgroup concerning prognosis even among patients within the same disease stage. It is difficult to comprehensively reflect the aggressiveness of a tumor only based on its size and location (14). Therefore, BM predictors based on tumor biology are required to reflect the biological characteristics of aggressiveness.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is a well-established molecular imaging technology that enables noninvasive quantification of tumor biological characteristics. The maximum standardized uptake value (SUVmax) is the most commonly used parameter for quantifying tumor FDG uptake. However, it only reflects the hottest voxel, and is therefore prone to high statistical noise, and does not represent the overall tumor metabolic activity (15). Recently, several studies have suggested that standardization of semiquantitative measurements, the ratio of FDG uptake in tumors to that in suitable reference regions, such as the mediastinal blood pool and normal liver, may be more accurate than SUVmax in predicting the prognosis for NSCLC (16-19). Furthermore, the volumetric PET parameters, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG), which have been investigated as measures of metabolic tumor burden are recognized as promising quantitative PET indices (20). Studies have shown that MTV and TLG are more significant prognostic predictors than tumor stage and SUVmax in NSCLC (21-23). However, whether these PET metabolic parameters can predict BM development in patients with surgically resected NSCLC has not yet been reported.
Therefore, the current study aimed to evaluate the predictive value of preoperative metabolic parameters derived from 18F-FDG PET/CT for BM development in patients with curatively resected NSCLC and to compare them with other predictors. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-385/rc).
Methods
Patients
All patients with newly diagnosed clinical stage I–IIIA NSCLC who underwent preoperative 18F-FDG PET/CT examination in our center between November 2012 and October 2021 were retrospectively reviewed. Patients with negative brain MRI or CT findings as part of their initial staging, who were treated with curative surgery for NSCLC with a negative surgical margin, had no history of other prior malignancies, and underwent complete follow-up information were eligible for the study. Patients were excluded if they had diabetes or liver disorders or had received neoadjuvant chemotherapy or radiotherapy before surgery. Patients’ demographic and clinicopathological variables were recorded at the time of diagnosis, including age, sex, smoking status, preoperative serum carcinoembryonic antigen (CEA) level, histology, and tumor stage.
Patients were followed up every 6 months for 2 years and annually thereafter. As clinically indicated, follow-up evaluations included medical history, physical examination, abdominal ultrasound, thoracic CT, and other necessary examinations. If the patients had suspicious symptoms and/or if any recurrence was detected, a contrast-enhanced brain MRI examination was performed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional board of The First Affiliated Hospital of Jinan University (No. KY-2021-075) and individual consent for this retrospective analysis was waived.
PET/CT acquisition
The identical approach was followed throughout every examination utilizing a GE Discovery PET/CT 690 scanner. Patients were instructed to fast for at least 6 hours before the examination and have serum glucose levels under 200 mg/dL (24). PET/CT images were obtained 50–70 min after administering 0.08–0.10 mCi/kg of 18F-FDG intravenously. An unenhanced CT scan was acquired during shallow breathing at 130 kV, 100–180 mA modulated using the GE AutomA technique. Utilizing three-dimensional (3D) time-of-flight (TOF) technology, PET data were obtained through 2-min scans for each bed position. The scanning range covered from the top of the skull to mid-thigh, and the scanning time was approximately 20 min for every patient. The PET data were attenuation-corrected using CT data and then reconstructed in terms of point spread function (PSF) together with TOF technology.
Measurement of PET parameters
The PET parameters of the tumors were analyzed using PET volume computer-assisted reading (PET VCAR) which is an automated segmentation system on the GE Advantage Workstation. By using an iterative adaptive segmentation algorithm, this software can find a threshold value that separates the target volume from the background tissue by weighting the SUVmax and mean SUV (SUVmean) within the target volume with a default weighting factor of 0.5 (25,26). Two experienced independent nuclear physicians analyzed the primary tumors, and one physician analyzed all cases after a 3-week washout period. By drawing a volume of interest (VOI), the SUVmax, MTV, and TLG of the primary tumor were measured automatically. The metabolic parameters of lymph node metastases were not measured because their number was small, and most had low metabolism. The liver SUVmean was automatically measured using PET VCAR by drawing a spherical reference region, 3 cm in diameter, placed in the right lobe of the liver. Furthermore, the arterial blood SUVmean was measured manually by drawing a minimum VOI of 5 mL at the descending thoracic aorta (27). The tumor-to-liver SUV ratio (TLR) was calculated by dividing the tumor SUVmax by the liver SUVmean. Subsequently, in the same way, the tumor-to-blood SUV ratio (TBR) was obtained by the ratio of tumor SUVmax to blood SUVmean.
Statistical analysis
Patients with BM as the first site of failure were classified into the BM-positive group, whereas those with extracranial recurrence as the initial site of failure or with no recurrence were classified into the BM-negative group. The primary outcomes were brain metastasis-free survival (BMFS) and overall survival (OS). BMFS was measured from the date of surgery to the date of BM as the first relapse site confirmed by brain imaging or the last date when the patient was known to be free of BM. OS was defined as the interval from the date of surgery to death from any cause or final follow-up.
All PET/CT parameters were tested for interobserver and intraobserver agreements using interclass correlation coefficient (ICC) analysis. The differences in PET parameter values between the BM-positive and BM-negative groups were analyzed using the Mann-Whitney U test. Receiver operating characteristic (ROC) curve analysis was used to analyze significant BM-related parameters to establish the optimal cut-off values for predicting BMFS. Patients were divided into two subgroups depending on the optimal cut-off values. The Kaplan-Meier method was used to estimate the BMFS and OS curves of the different subgroups, and the log-rank test was used to compare them. Using univariate and multivariate Cox regression analyses, the risk factors for BM development, comprising various PET measures and other clinical characteristics, were discovered. To incorporate more potential risk factors, each factor with a P<0.1 in the univariate analysis was further analyzed by the multivariate analysis. the P value was a one-sided test. All data were analyzed using SPSS software version 16.
Results
Patient characteristics
A total of 128 patients (71 men and 57 women; mean age, 63 years; range, 32–80 years) were enrolled in this study, of whom 102 (79.7%) were adenocarcinomas, 22 (17.2%) were squamous cell carcinomas, 3 (2.3%) were large cells, and 1 (0.8%) was adenosquamous. Thirty-one (24.2%) patients had preoperative MRI and 97 (75.8%) had preoperative CT to exclude BM. All patients had retrospectively staged again with American Joint Committee on Cancer (AJCC) 8th edition for consistency (28). The tumor stages were IA in 80 (62.5%) patients, IB in 16 (12.5%), IIA in 5 (3.9%), IIB in 15 (11.7%), and IIIA in 12 (9.4%) patients. A flow diagram of the study is shown in Figure 1. The mean time between 18F-FDG PET/CT examination and surgery was 7±2 days (range, 1–20 days). All patients underwent curative-intent surgery plus systematic lymph node dissection, and 26 (20.3%) also received adjuvant chemotherapy for four to six cycles following radical resection. The chemotherapy regimens included carboplatin- or cisplatin-based regimens in conjunction with paclitaxel, docetaxel, pemetrexed, or gemcitabine. Table 1 summarizes the demographics and clinical features of patient.
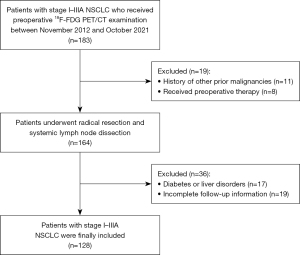
Table 1
| Characteristics | Values |
|---|---|
| Age (years), median [range] | 63 [32–80] |
| Sex, n (%) | |
| Male | 71 (55.5) |
| Female | 57 (44.5) |
| Smoking history, n (%) | |
| Never | 97 (75.8) |
| Ever/current | 31 (24.2) |
| Histology, n (%) | |
| Adenocarcinoma | 102 (79.7) |
| Squamous cell carcinoma | 22 (17.2) |
| Large cell carcinoma | 3 (2.3) |
| Adenosquamous carcinoma | 1 (0.8) |
| T stage, n (%) | |
| 1 | 89 (69.5) |
| 2 | 32 (25.0) |
| 3 | 5 (3.9) |
| 4 | 2 (1.6) |
| N stage, n (%) | |
| 0 | 108 (84.4) |
| 1 | 10 (7.8) |
| 2 | 10 (7.8) |
| TNM stage (AJCC 8th edition), n (%) | |
| IA | 80 (62.5) |
| IB | 16 (12.5) |
| IIA | 5 (3.9) |
| IIB | 15 (11.7) |
| IIIA | 12 (9.4) |
| Type of surgery, n (%) | |
| Lobectomy | 121 (94.5) |
| Wedge resection | 7 (5.5) |
| Adjuvant therapy, n (%) | |
| No | 102 (79.7) |
| Chemotherapy | 26 (20.3) |
TNM, Tumor Node Metastasis; AJCC, American Joint Committee on Cancer.
BMFS and OS analysis
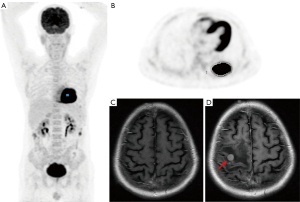
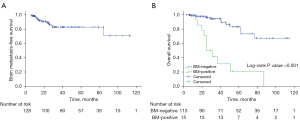
The median follow-up duration was 23.4 months (range, 5.2–113.2 months). A total of 22 (17.2%) patients had died, and 106 (82.8%) patients were still alive at the time of analysis. Thirty-five patients (27.3%) experienced recurrence; of these, 20 patients (15.6%) developed extracranial recurrence as the initial site of failure, while 15 patients (11.7%) experienced BM as the first relapse location. A representative clinical case is shown in Figure 2. The median time of BM diagnosis after surgery was 14.2 months (range, 5.2–84.6 months), and the cumulative rates of BM over the course of 1, 2, and 5 years were 4.5%, 10.5%, and 17.5%, respectively (Figure 3A). Patients with positive BM had significantly lower OS than those with negative (P<0.001) (Figure 3B).
Interobserver and intraobserver agreement of PET parameters
The interobserver and intraobserver agreements in SUVmax of the primary tumors were excellent, with ICC values of 1.000. The ICC values of 1.000 were also found for SUVmean of the liver, as the VOI on the right lobe of the liver was generated automatically by PET VCAR. Excellent interobserver and intraobserver agreements were also found for other PET parameters, with ICC values ranging from 0.957 to 0.998. Therefore, the results from Physician 1 were used for further analysis.
PET parameters and ROC analysis
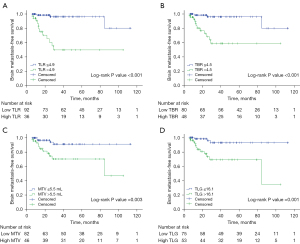
Table 2 describes the mean and optimal cut-off values of various parameters as determined by ROC curves. SUVmax, TLR, TBR, MTV, and TLG were significantly higher in the BM-positive group than in the BM-negative group (P<0.001). The optimal cut-off values for BMFS derived from the area under the curve (AUC) data were 7.7, 4.9, and 4.5 for SUVmax, TLR, and TBR, and 5.5 mL and 16.1 for MTV and TLG, respectively. Using these derived optimal cut-off points, patients with high PET parameters at the primary tumor level had significantly shorter BMFS (P<0.05). The Kaplan-Meier curves with dichotomized values of TLR, TBR, MTV, and TLG are shown in Figure 4.
Table 2
| Parameter | Total (mean ± SD) |
BM negative (mean ± SD) | BM positive (mean ± SD) | Pa | AUC (95% CI) | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
| SUVmax | 6.8±5.5 | 6.0±4.7 | 13.1±7.3 | <0.001 | 0.845 (0.764–0.925) | 7.7 | 0.929 | 0.728 |
| TLR | 3.7±2.9 | 3.3±2.7 | 7.0±2.9 | <0.001 | 0.853 (0.771–0.936) | 4.9 | 0.929 | 0.798 |
| TBR | 4.2±3.8 | 3.8±3.7 | 7.6±3.4 | <0.001 | 0.832 (0.745–0.920) | 4.5 | 0.929 | 0.693 |
| MTV (mL) | 9.0±19.8 | 8.0±20.0 | 17.5±16.6 | <0.001 | 0.788 (0.665–0.911) | 5.5 | 0.857 | 0.702 |
| TLG | 50.0±138.9 | 39.1±127.0 | 138.7±197.1 | <0.001 | 0.845 (0.762–0.929) | 16.1 | 0.929 | 0.649 |
a, Mann-Whitney U test. PET, positron emission tomography; SUVmax, maximum standardized uptake value; TLR, tumor-to-liver SUV ratio; TBR, tumor-to-blood SUV ratio; MTV, metabolic tumor volume; TLG, total lesion glycolysis; SD, standard deviation; AUC, area under the curve; CI, confidence interval.
Risk factors of BM
Table 3 summarizes the results of the univariate and multivariate analyses. High T stage (T2–4), TNM stage (II–IIIA), serum CEA level >3.76 ng/mL, SUVmax >7.7, TLR >4.9, TBR >4.5, MTV >5.5 mL, and TLG >16.1 were significantly associated with an increased risk of developing brain relapses in univariate analysis (all P<0.05). Only high TLR [hazard ratio (HR) =10.712; 95% confidence interval (CI): 2.958–38.801; P<0.001] and high MTV (HR =3.150; 95% CI: 0.964–10.293; P=0.020) were risk factors for BM according to multivariate analysis, even after adjusting for T stage, N stage, TNM stage, serum CEA level, and other PET parameters.
Table 3
| Factors | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (≤60 vs. >60 years) | 1.108 | 0.378–3.248 | 0.851 | – | – | – | |
| Sex (female vs. male) | 2.290 | 0.760–6.898 | 0.141 | – | – | – | |
| Smoking (never vs. ever/current) | 1.467 | 0.490–4.390 | 0.493 | – | – | – | |
| Histology (adenocarcinoma vs. non-adenocarcinoma) | 2.194 | 0.495–9.735 | 0.301 | – | – | – | |
| Adjuvant therapy (no vs. yes) | 0.577 | 0.195–1.706 | 0.320 | – | – | – | |
| Surgical approaches (wedge vs. lobectomy) | 0.044 | 0.000–459.047 | 0.509 | – | – | – | |
| T stage (T1 vs. T2–4) | 2.941 | 1.058–8.171 | 0.039 | – | – | – | |
| N stage (N0 vs. N1–2) | 2.902 | 0.909–9.269 | 0.072 | – | – | – | |
| TNM stage (I vs. II–IIIA) | 3.182 | 1.145–8.842 | 0.026 | – | – | – | |
| CEA level (≤3.76 vs. >3.76 ng/mL) | 3.309 | 1.167–9.385 | 0.024 | – | – | – | |
| SUVmax (≤7.7 vs. >7.7) | 11.036 | 3.077–39.577 | 0.001 | – | – | – | |
| TLR (≤4.9 vs. >4.9) | 13.285 | 3.729–47.327 | <0.001 | 10.712 | 2.958–38.801 | <0.001 | |
| TBR (≤4.5 vs. >4.5) | 9.639 | 2.689–34.551 | 0.001 | – | – | – | |
| MTV (≤5.5 vs. >5.5 mL) | 4.736 | 1.506–14.889 | 0.008 | 3.150 | 0.964–10.293 | 0.020 | |
| TLG (≤16.1 vs. >16.1) | 6.801 | 1.911–24.197 | 0.003 | – | – | – | |
HR, hazard ratio; CI, confidence interval; TNM, Tumor Node Metastasis; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; TLR, tumor-to-liver SUV ratio; TBR, tumor-to-blood SUV ratio; MTV, metabolic tumor volume; TLG, total lesion glycolysis.
Discussion
The results of this study suggest that the metabolic parameters of the primary tumor derived from preoperative 18F-FDG PET may have a potential in predicting BM development in patients with surgically resected NSCLC. The median BMFS was shorter in patients with high SUVmax, TLR, TBR, MTV, and TLG. In addition, multivariate analysis showed that TLR and MTV were significant predictors of a higher risk of relapse with BM in patients with NSCLC who had undergone curative surgery, even after adjusting for tumor stage, well-known clinicopathological predictive factors, and other PET parameters.
BM is a common complication in patients with early-stage NSCLC after receiving curative treatment. Previous studies found that the incidence of BM ranged from 6.2% to 20.9%, and the median BMFS was 10.0–11.43 months in surgically resected NSCLC patients (1-3). Most BM occurs within 2 years of their diagnosis. Our research results are consistent with these observations, with a total BM incidence of 11.7%, a median BMFS of 14.2 months, and a 2-year BM actuarial rate of 10.5%. Additionally, we found that the OS of the BM-positive group was considerably lower than that of the BM-negative group, which is in line with earlier research (29).
The tumor-to-reference region activity ratio, which is the normalized value of PET parameters by blood pool or liver SUV, has been shown in previous studies to be a more accurate predictor of patient prognosis than the tumor SUVmax (16-19). SUV-based parameters are prone to be affected by certain factors, and normalization using normal tissue uptake may reduce the effect of these individual biases. In a study by Shiono et al. (17) which included 141 patients with pathological stage I lung adenocarcinoma, the TLR was a significant predictive and reproducible factor for recurrence. In another study by Shin et al. (18), the TBR was an independent predictor of recurrence in 77 patients with stage I–III NSCLC who underwent curative surgery. However, none of these studies specifically focused on the development of BM. In the present study, we comprehensively evaluated various PET parameters at different angles and estimated the optimal cut-off points to identify clinically predictive markers for BM. Similarly, we found that TLR was better than other PET parameters in predicting an increased risk of relapse with BM and was an independent predictor of BM based on multivariate analysis.
The volumetric PET parameters, MTV and TLG, can help better estimate tumor burden and biological aggressiveness. MTV is a measure of the tumor volume showing FDG uptake over a minimal threshold with the intention to exclude background activity and quantify the entire tumor burden, whereas TLG is representative of both tumor volume and metabolic activity. Previous studies have shown that MTV and TLG are significant and independent predictive factors of progression in patients with NSCLC (21-23). In the present study, we found that the MTV and TLG of the primary tumor were significantly associated with the risk of developing BM as the first failure in the univariate analysis, and MTV remained significant even after adjusting for T stage, TNM stage, serum CEA values, and other PET parameters in the multivariate analysis.
Several studies have reported that the T, N, and TNM stages of NSCLC are associated with BM incidence (10,11). However, with disease development, the accuracy of the tumor stage as a surrogate for tumor burden decreases because of a broad spectrum of disease severities represented by only a few different stages (14). In the present study, we found that higher T and TNM stages were associated with a higher risk of BM; however, they were not significant predictors after adjusting for PET parameters in the multivariate analysis. This finding is consistent with previous studies showing that 18F-FDG metabolic parameters may provide better prognostic information than the tumor stage (30,31). Furthermore, other prognostic factors such as age, sex, histology, and serum CEA levels have also been reported to be related to the risk of BM in NSCLC; however, the results are inconsistent (32). In the current study, we only found that the occurrence rate of BM was associated with high serum CEA levels.
The National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) clinical practice guidelines recommend intracranial imaging only for neurologically symptomatic patients after surgical resection (6,7). However, this would likely lead to occult BM in patients with NSCLC without neurological symptoms being overlooked. Ando et al. (33) analyzed 46 NSCLC patients with BM and found that 29 patients (63%) were asymptomatic when diagnosed. Furthermore, previous studies have shown that early detection of asymptomatic BM is linked to decreased morbidity and mortality (34-36). A retrospective study by Gao et al. (34) demonstrated that patients with neurological symptoms at the time of BM diagnosis had shorter progression-free survival and OS compared with asymptomatic patients. Therefore, regular brain imaging follow-up to detect BM are important and should be performed based on the risks of BM rather than the emergence of neurological signs or symptoms. Our study found that higher TLR and MTV of the primary tumor were significantly associated with the risk of developing BM. These findings may help physicians identify subgroups of NSCLC patients with different risks of BM and determine individualized surveillance strategies.
The current study has some limitations. First, the study design was retrospective, and the sample size was relatively small. Further prospective studies, including larger homogeneous patient cohorts, more parameters such as clinicopathological factors and mutation state of molecular biomarkers, and longer follow-up time, are required to comprehensively validate our results. Second, although MTV and TLG have advantages in measuring the tumor metabolic burden, the optimal segmentation method for calculating these volumetric parameters is still under debate. In this study, the MTV and TLG were automatically computed by PET VCAR using an iterative adaptive segmentation algorithm. This method has an advantage over fixed-threshold methods in accurately delineating the target volume according to individual metabolic activity (37). Finally, the metabolic parameters of lymph node metastases were not included in this study because their number was small, and most had low metabolism. Furthermore, the N stage was also not a risk factor for BM development in the univariate analysis.
Conclusions
The preoperative metabolic parameters of the primary tumor, as measured by TLR and MTV on 18F-FDG PET, are independent predictors of BM development as the first relapse site in patients with surgically resected NSCLC. Therefore, adding measurements of tumor metabolic parameters may help stratify the risk of BM development and determine individualized surveillance strategies. These results must be validated in a multicenter prospective study with a larger homogeneous patient cohort.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-385/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-385/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional board of The First Affiliated Hospital of Jinan University (No. KY-2021-075) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hubbs JL, Boyd JA, Hollis D, Chino JP, Saynak M, Kelsey CR. Factors associated with the development of brain metastases: analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer 2010;116:5038-46. [Crossref] [PubMed]
- Zhang Y, Zheng D, Xie J, Li Y, Wang Y, Li C, Xiang J, Zhang Y, Hu H, Sun Y, Chen H. Development and Validation of Web-Based Nomograms to Precisely Predict Conditional Risk of Site-Specific Recurrence for Patients With Completely Resected Non-small Cell Lung Cancer: A Multiinstitutional Study. Chest 2018;154:501-11. [Crossref] [PubMed]
- Sun F, Chen Y, Chen X, Sun X, Xing L. CT-based radiomics for predicting brain metastases as the first failure in patients with curatively resected locally advanced non-small cell lung cancer. Eur J Radiol 2021;134:109411. [Crossref] [PubMed]
- Peters S, Bexelius C, Munk V, Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 2016;45:139-62. [Crossref] [PubMed]
- Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, Weinberg JS, Zadeh G, Schiff D. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol 2022;40:492-516. [Crossref] [PubMed]
- National Comprehensive Cancer Network Organization. Guidelines in Oncology: non-small cell lung cancer V1. 2023. [accessed January 3, 2023]. Available at: https://www.nccn.org/
- Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, Escriu C, Peters S, Committee EG. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, Chang SD. Brain metastases. Nat Rev Dis Primers 2019;5:5. [Crossref] [PubMed]
- Hwang JK, Page BJ, Flynn D, Passmore L, McCaul E, Brady J, Yang IA, Marshall H, Windsor M, Bowman RV, Naidoo R, Guan T, Philpot S, Blake ME, Fong KM. Validation of the Eighth Edition TNM Lung Cancer Staging System. J Thorac Oncol 2020;15:649-54.
- Won YW, Joo J, Yun T, Lee GK, Han JY, Kim HT, Lee JS, Kim MS, Lee JM, Lee HS, Zo JI, Kim S. A nomogram to predict brain metastasis as the first relapse in curatively resected non-small cell lung cancer patients. Lung Cancer 2015;88:201-7. [Crossref] [PubMed]
- Zhang F, Zheng W, Ying L, Wu J, Wu S, Ma S, Su D. A Nomogram to Predict Brain Metastases of Resected Non-Small Cell Lung Cancer Patients. Ann Surg Oncol 2016;23:3033-9. [Crossref] [PubMed]
- Ding X, Dai H, Hui Z, Ji W, Liang J, Lv J, Zhou Z, Yin W, He J, Wang L. Risk factors of brain metastases in completely resected pathological stage IIIA-N2 non-small cell lung cancer. Radiat Oncol 2012;7:119. [Crossref] [PubMed]
- Zhang Q, Cai XW, Feng W, Yu W, Fu XL. Risk factors of brain metastases as initial failure in completely resected stage IIIA(N2) non-small cell lung cancer. Ann Transl Med 2020;8:374. [Crossref] [PubMed]
- UyBico SJ. Wu CC, Suh RD, Le NH, Brown K, Krishnam MS. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 2010;30:1163-81. [Crossref] [PubMed]
- Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging 2017;44:17-31. [Crossref] [PubMed]
- Li XF, Shi YM, Niu R, Shao XN, Wang JF, Shao XL, Zhang FF, Wang YT. Risk analysis in peripheral clinical T1 non-small cell lung cancer correlations between tumor-to-blood standardized uptake ratio on 18F-FDG PET-CT and primary tumor pathological invasiveness: a real-world observational study. Quant Imaging Med Surg 2022;12:159-71. [Crossref] [PubMed]
- Shiono S, Abiko M, Okazaki T, Chiba M, Yabuki H, Sato T. Positron emission tomography for predicting recurrence in stage I lung adenocarcinoma: standardized uptake value corrected by mean liver standardized uptake value. Eur J Cardiothorac Surg 2011;40:1165-9. [Crossref] [PubMed]
- Shin S, Pak K, Kim IJ, Kim BS, Kim SJ. Prognostic Value of Tumor-to-Blood Standardized Uptake Ratio in Patients with Resectable Non-Small-Cell Lung Cancer. Nucl Med Mol Imaging 2017;51:233-9. [Crossref] [PubMed]
- Huang J, Huang L, Zhou J, Duan Y, Zhang Z, Wang X, Huang P, Tan S, Hu P, Wang J, Huang M. Elevated tumor-to-liver uptake ratio (TLR) from 18F-FDG-PET/CT predicts poor prognosis in stage IIA colorectal cancer following curative resection. Eur J Nucl Med Mol Imaging 2017;44:1958-68. [Crossref] [PubMed]
- Du S, Sun H, Gao S, Xin J, Lu Z. Metabolic parameters with different thresholds for evaluating tumor recurrence and their correlations with hematological parameters in locally advanced squamous cell cervical carcinoma: an observational 18F-FDG PET/CT study. Quant Imaging Med Surg 2019;9:440-52. [Crossref] [PubMed]
- Hyun SH, Choi JY, Kim K, Kim J, Shim YM, Um SW, Kim H, Lee KH, Kim BT. Volume-based parameters of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann Surg 2013;257:364-70. [Crossref] [PubMed]
- Park SY, Cho A, Yu WS, Lee CY, Lee JG, Kim DJ, Chung KY. Prognostic value of total lesion glycolysis by 18F-FDG PET/CT in surgically resected stage IA non-small cell lung cancer. J Nucl Med 2015;56:45-9. [Crossref] [PubMed]
- Seban RD, Mezquita L, Berenbaum A, Dercle L, Botticella A, Le Pechoux C, Caramella C, Deutsch E, Grimaldi S, Adam J, Ammari S, Planchard D, Leboulleux S, Besse B. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imaging 2020;47:1147-57. [Crossref] [PubMed]
- Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. [Crossref] [PubMed]
- Shang J, Ling X, Zhang L, Tang Y, Xiao Z, Cheng Y, Guo B, Gong J, Huang L, Xu H. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2016;43:1945-53. [Crossref] [PubMed]
- Shang J, Tan Z, Cheng Y, Tang Y, Guo B, Gong J, Ling X, Wang L, Xu H. A method for evaluation of patient-specific lean body mass from limited-coverage CT images and its application in PERCIST: comparison with predictive equation. EJNMMI Phys 2021;8:12. [Crossref] [PubMed]
- Hofheinz F, Li Y, Steffen IG, Lin Q, Lili C, Hua W, van den Hoff J, Zschaeck S. Confirmation of the prognostic value of pretherapeutic tumor SUR and MTV in patients with esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging 2019;46:1485-94. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack VInternational Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Ernani V, Stinchcombe TE. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J Oncol Pract 2019;15:563-70. [Crossref] [PubMed]
- Tosi D, Pieropan S, Cattoni M, Bonitta G, Franzi S, Mendogni P, Imperatori A, Rotolo N, Castellani M, Cuzzocrea M, Schiorlin I, Casagrande S, De Palma D, Nosotti M, Dominioni L. Prognostic Value of 18F-FDG PET/CT Metabolic Parameters in Surgically Treated Stage I Lung Adenocarcinoma Patients. Clin Nucl Med 2021;46:621-6. [Crossref] [PubMed]
- Chou HP, Lin KH, Huang HK, Lin LF, Chen YY, Wu TH, Lee SC, Chang H, Huang TW. Prognostic value of positron emission tomography in resected stage IA non-small cell lung cancer. Eur Radiol 2021;31:8021-9. [Crossref] [PubMed]
- An N, Jing W, Wang H, Li J, Liu Y, Yu J, Zhu H. Risk factors for brain metastases in patients with non-small-cell lung cancer. Cancer Med 2018;7:6357-64. [Crossref] [PubMed]
- Ando T, Kage H, Saito M, Amano Y, Goto Y, Nakajima J, Nagase T. Early stage non-small cell lung cancer patients need brain imaging regardless of symptoms. Int J Clin Oncol 2018;23:641-6. [Crossref] [PubMed]
- Gao YK, Kuksis M, Id Said B, Chehade R, Kiss A, Tran W, Sickandar F, Sahgal A, Warner E, Soliman H, Jerzak KJ. Treatment Patterns and Outcomes of Women with Symptomatic and Asymptomatic Breast Cancer Brain Metastases: A Single-Center Retrospective Study. Oncologist 2021;26:e1951-e61. [Crossref] [PubMed]
- Hanzly M, Abbotoy D, Creighton T, Diorio G, Mehedint D, Murekeyisoni C, Attwood K, Kauffman E, Fabiano AJ, Schwaab T. Early identification of asymptomatic brain metastases from renal cell carcinoma. Clin Exp Metastasis 2015;32:783-8. [Crossref] [PubMed]
- Venur VA, Chukwueke UN, Lee EQ. Advances in Management of Brain and Leptomeningeal Metastases. Curr Neurol Neurosci Rep 2020;20:26. [Crossref] [PubMed]
- Xu W, Yu S, Ma Y, Liu C, Xin J. Effect of different segmentation algorithms on metabolic tumor volume measured on 18F-FDG PET/CT of cervical primary squamous cell carcinoma. Nucl Med Commun 2017;38:259-65. [Crossref] [PubMed]


