
A case of primary malignant melanoma of the female genital tract involving both the cervix and vagina
Introduction
Malignant melanoma (MM) is a cancer originating from melanocytes, which are mainly located in the basal layer of the epithelium, and can occur in different parts or tissues such us the skin and mucosal membranes. The annual incidence of genitourinary melanoma in women is 1.74 per 1 million (1). The most common site of genital tract melanoma (GTMM) is the vulva and vagina. Involvement of the cervix, uterus, and ovary is extremely rare. According to statistics, vaginal melanoma accounts for 1–5% of all vaginal malignancies (2), and the incidence of cervical melanoma is 5 times lower than that of vaginal or vulvar melanoma (3). Primary MM of the vagina and cervix is sporadic. The peak incidence age for female GTMM is 50–60 years old, and the median age is 58 years (4,5). There is no established standard of treatment for this disease. Radiotherapy, chemotherapy, and immunotherapy have been be used as adjuvant therapy in combination with various surgical treatments, including radical hysterectomy, vaginectomy, and pelvic lymphadenectomy. However, the scope of the surgery remains unclear (2,6). The disease has a poor prognosis, with almost half of the patients dying within 12 months of initial diagnosis, and is prone to recurrence. Therefore, improving the rate of diagnosis for MM remains an urgent clinical need.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
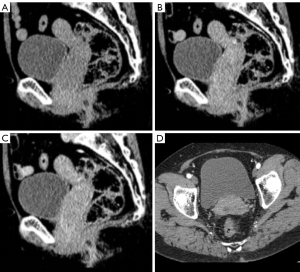
The patient was a 65-year-old female who experienced menarche at 15 years of age and gestation 1 parturition 1. The patient’s main complaint was irregular vaginal bleeding for 5 months, involving a small amount of dark red blood, for which there was no diagnosis or treatment. Previous cervical smear examination was negative for interepithelial lesions and neoplasia. Physical examination showed no dark spots, nevus, erosion, or ulcer on the skin and mucosa of the whole body. There was no palpable enlargement of the superficial lymph nodes. Gynecologic examination revealed that the cervix was abnormal in gross appearance, and a mass with a diameter of 4 cm was seen in the cervix, with a brittle texture and tendency to bleed. Laboratory tests did not show any significant abnormalities. Tumor markers, CA125, CA153, CA199, carcinoembryonic antigen (CEA), and alpha-fetoprotein (AFP) were negative. Pelvic computed tomography (CT) revealed thickening of the cervical and vaginal walls with soft tissue mass shadow, with a CT value of about 32 Hounsfield units (HU) and an unclear boundary. The lesion had invaded the upper two-thirds of the vagina downward, and contrast-enhanced dual-phase CT values were approximately 72 and 67 HU. There were no evident enlarged lymph nodes in the parauterine or pelvic cavity (Figure 1). Pelvic magnetic resonance imaging (MRI) findings included a mass of abnormal signal in the right anterior wall of the uterine cervix and the middle and upper part of the vagina, mainly located in the anterior wall and invading into the periphery. The lesion invaded downward into the upper two-thirds of the vagina and approached the lower one-third. The lesion showed equal hyperintensity on T1-weighted imaging (T1WI), hyperintensity on T2-weighted imaging (T2WI) with a clear border and an estimated size of 5.5 cm × 4.9 cm × 2.8 cm, and obvious hyperintensity on diffusion-weighted imaging (DWI) with an unclear border. The apparent diffusion coefficient (ADC) value was approximately 0.49×10−3 mm2/s. In the contrast-enhanced phase, the mass showed early significant enhancement that was rapidly washed out with marginal enhancement (Figure 2A-2J). Preoperative imaging failed to provide a clear diagnosis. A cervical mucosal biopsy was performed at the gynecological clinic: several pieces of gray-red tissue were taken from the cervix, and under microscopy, the tumor cells appeared polymorphic, polygonal, and spindle-shaped (Figure 2K,2L). Immunohistochemical findings were as follows: S-100 protein (+), human melanoma black-45 (+), melan antigen (+), cytokeratin (–), vimentin (+), and Ki-67 proliferation index 50% (Figure 3). The combined findings of pathology and immunohistochemistry led to a diagnosis of melanoma, with an International Federation of Gynecology and Obstetrics (FIGO) grade of IIB–III. However, it was still impossible to identify whether the primary site was the vulva or the cervix. After three cycles of chemotherapy with albumin-bound paclitaxel, carboplatin, and bevacizumab, the tumor size showed a decreasing trend, and shrank to about 5.5 cm × 4.4 cm × 2.5 cm in size. After 3 months of regular follow-up, the patient was alive with no obvious metastasis.


Discussion
The etiology and pathogenesis of melanoma are poorly understood, and its clinical manifestations are nonspecific. Cervical melanoma is clinically characterized by irregular vaginal bleeding, postmenopausal vaginal bleeding, and increased vaginal discharge (7). Cervical pigmentation can be observed via colposcopy. Between 2% and 9% of melanomas have no clinical or histopathological evidence of hyperpigmentation (8), and the clinical manifestations and diffusion model of melanoma for the cervix are highly similar to those of other epithelial malignant tumors and is difficult to differentiate from other histological types of cervical cancer. MM of the cervix is generally considered to be a human papillomavirus (HPV)-independent tumor. However, some studies have found that HPV may play an auxiliary role in the development of melanoma (9,10). In the early stage of MM, distant metastases such as to the liver, lung, or brain can occur. Comprehensive examination should be performed to exclude other primary MM and distant metastases of this tumor. It has also been claimed that melanocytes of the cervix are derived from Schwann cells that migrate from the neural crest, and during this process, these cells can give rise to rare morphological entities such as blue nevus and benign melanosis (11,12), a condition considered by many to be a precursor to cervical MM.
As primary MM of the cervix is extremely rare, it is critical to rule out primary melanoma from other positions before making a diagnosis of primary cervical MM. The cervix is usually involved as a secondary site, either in the recurrence of a vaginal or vulvar tumor or as a primary hematologic metastasis from another part of the body (13). The literature (14) suggests that cervical melanoma typically involves the vagina, and vaginal MM frequently occurs in the anterior wall of the lower vaginal segment. In our case, the lesion appeared as a large mass in the cervix and the upper part of the vagina, and the lesion had locally invaded the right lateral cervical stroma and the anterior vaginal wall. We presumed that the lesion originated from the cervix and had invaded downward to the vaginal wall. Nevertheless, in terms of prognosis, early detection of the tumor is more meaningful than is discerning the site of origin.
The only literature concerning cervical melanoma is in the form of case reports, the majority of which are clinicopathological analyses, and imaging studies are rare. In our case, the cervical mucosa was continuous and smooth, and the fibrous stroma of the right lateral wall of the cervix was locally thinned and collapsed, the lesion had grown backward to the mucosal surface, and the center of the lesion was outside the cervical canal. The possibility of an uncommon lesion derived from mucosal epithelial cells was considered. Melanin particles are paramagnetic substances, which can shorten the relaxation time of T1WI and T2WI, and thus their MRI manifestations are specific; that is, the primary tumor and metastatic tumor of melanoma appear isointense and hyperintense signal on T1WI and hypointense on T2WI. In our case, both T1WI and T2WI showed a slightly hyperintense signal, which is rare in common cervical tumors such as cervical cancer, and may be related to the proportion of intracellular melanin particles. On the DWI sequence, the diffusion limitation of the lesion was significantly aggravated, suggesting that the tumor cells were closely arranged, and thus the possibility of malignancy could not be excluded. The lesions were obviously enhanced in the early stage and rapidly washed out in the venous phase and delayed phase. These imaging findings suggested that although the lesion was a malignant lesion in the cervix, it did not have the same imaging manifestations as those of general cervical cancer, and the possibility of a rare lesion type needed to be considered. Squamous cell carcinoma is more common in cervical cancer, the nodules or masses have a slightly longer T1 and a slightly longer T2 signal, the cervical mucosa is not continuous, and the enhancement is uneven. Diffuse large B-cell lymphoma (DLBCL) is the most common type of cervical lymphoma and has a homogeneous signal with mild-to-moderate enhancement. It is often accompanied by abdominal and pelvic multiple lymph node enlargement and fusion. These imaging manifestations are similar to those of cervical cancer, making differentiation challenging. The imaging findings of the patient in this case and those of case reports from the literature suggest that the imaging of cervical melanoma has certain characteristics but no specificity, and thus the diagnosis should be made with reference to pathological and immunohistochemical results. When the above manifestations are not consistent with those of common cervical lesions, MM should be considered.
The 2017 American Joint Committee on Cancer (AJCC) skin melanoma staging system can be used to assess the recurrence and survival of vulvar and vaginal melanoma. Meanwhile, primary melanomas of the uterine cervix are primarily evaluated according to the 2019 FIGO staging system (6,13,14). Currently, there is no general recommendation for the therapeutic schedule of primary female GTMM. For early-stage vaginal melanoma, we do not recommend radical surgery and instead support wide local excision (WLE) as the primary treatment (4,14). The treatment of cervical melanoma is mainly based on the treatment principles of cervical cancer. Radical hysterectomy and pelvic lymphadenectomy are often selected for early-stage tumors, and para-aortic lymphadenectomy is also an option. For advanced tumors, pelvic primary radiotherapy is usually preferred. Hadron therapy has recently been reported as a potential therapeutic option for mucosal melanomas, and one study (15) reported that radiotherapy combined with immunotherapy effectively was a favorable approach for the treatment of vaginal melanoma. Some studies advocate adjuvant pelvic radiotherapy for patients with lymph nodes coexisting with borderline-positive or histologically positive risk factors (6,11). Postoperative adjuvant chemotherapy has been clinically demonstrated to provide a survival benefit for resectable mucosal melanoma. For the immunotherapy and biological therapy of female GTMM, some patients may benefit from long-term remission (16), but large-scale studies are still needed to confirm this. Some research (6,17,18) suggests that anti–programmed cell death protein 1 therapy is more effective for improving overall survival in patients with advanced or recurrent GTMM. In summary, the treatment approach tends not to be standardized and is rather individualized according to the characteristics of the disease and the patient.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-979/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vyas R, Thompson CL, Zargar H, Selph J, Gerstenblith MR. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol 2016;75:144-50. [Crossref] [PubMed]
- Tasaka R, Fukuda T, Wada T, Kawanishi M, Imai K, Kasai M, Hashiguchi Y, Ichimura T, Yasui T, Sumi T. A retrospective clinical analysis of 5 cases of vaginal melanoma. Mol Clin Oncol 2017;6:373-6. [Crossref] [PubMed]
- Pusceddu S, Bajetta E, Carcangiu ML, Formisano B, Ducceschi M, Buzzoni R. A literature overview of primary cervical malignant melanoma: an exceedingly rare cancer. Crit Rev Oncol Hematol 2012;81:185-95. [Crossref] [PubMed]
- Huang Q, Huang H, Wan T, Deng T, Liu J. Clinical outcome of 31 patients with primary malignant melanoma of the vagina. J Gynecol Oncol 2013;24:330-5. [Crossref] [PubMed]
- Kechagias KS, Zafeiri M, Katsikas Triantafyllidis K, Kyrtsonis G, Geropoulos G, Lyons D, Burney Ellis L, Bowden S, Galani A, Paraskevaidi M, Kyrgiou M. Primary Melanoma of the Cervix Uteri: A Systematic Review and Meta-Analysis of the Reported Cases. Biology (Basel) 2023.
- Min A, Fu A, Huang M, Wang H, Chen H. Primary Malignant Melanoma of the Cervix: An Integrated Analysis of Case Reports and Series. Front Oncol 2022;12:913964. [Crossref] [PubMed]
- Yin C, Yang A, Zhang Y, Tao L, Zou H, Ren Y, Liang W, Jiang J, Zhao J, Zhang W, Li F, Jia W. Primary Cervical Malignant Melanoma: 2 Cases and a Literature Review. Int J Gynecol Pathol 2019;38:196-203. [Crossref] [PubMed]
- Wee E, Wolfe R, Mclean C, Kelly JW, Pan Y. Clinically amelanotic or hypomelanotic melanoma: Anatomic distribution, risk factors, and survival. J Am Acad Dermatol 2018;79:645-651.e4. [Crossref] [PubMed]
- Rohwedder A, Slominski A, Wolff M, Kredentser D, Carlson JA. Epidermodysplasia verruciformis and cutaneous human papillomavirus DNA, but not genital human papillomavirus DNAs, are frequently detected in vulvar and vaginal melanoma. Am J Dermatopathol 2007;29:13-7. [Crossref] [PubMed]
- Dréau D, Culberson C, Wyatt S, Holder WD Jr. Human papilloma virus in melanoma biopsy specimens and its relation to melanoma progression. Ann Surg 2000;231:664-71. [Crossref] [PubMed]
- Singh N, Tripathi R, Mala YM. Primary malignant melanoma of uterine cervix with probable origin from benign cervical melanosis. BMJ Case Rep 2013;2013:bcr2013010042. [Crossref] [PubMed]
- Eniu DT, Staicu A, Şomcutian O, Buiga R, Albu C, Goidescu IG, Chiorean AR, Nistor-Ciurba CC. Blue nevus-like melanoma of the uterine cervix. Case report and review of the literature. Rom J Morphol Embryol 2019;60:1317-21.
- Pang Y, Yuan H, Ren A, Zhang S, Liu P. Primary malignant melanoma of the female genital tract synchronously involving the vulva and uterine cervix: A case report. Medicine (Baltimore) 2019;98:e16366. [Crossref] [PubMed]
- Wang D, Xu T, Zhu H, Dong J, Fu L. Primary malignant melanomas of the female lower genital tract: clinicopathological characteristics and management. Am J Cancer Res 2020;10:4017-37.
- Cuccia F, D'Alessandro S, Blasi L, Chiantera V, Ferrera G. The Role of Radiotherapy in the Management of Vaginal Melanoma: A Literature Review with a Focus on the Potential Synergistic Role of Immunotherapy. J Pers Med 2023;13:1142. [Crossref] [PubMed]
- Gellrich FF, Schmitz M, Beissert S, Meier F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma-An Update. J Clin Med 2020;9:223. [Crossref] [PubMed]
- Indini A, Di Guardo L, Cimminiello C, Lorusso D, Raspagliesi F, Del Vecchio M. Investigating the role of immunotherapy in advanced/recurrent female genital tract melanoma: a preliminary experience. J Gynecol Oncol 2019;30:e94. [Crossref] [PubMed]
- Liao KL, Watt KD, Protin T. Different mechanisms of CD200-CD200R induce diverse outcomes in cancer treatment. Math Biosci 2023;365:109072. [Crossref] [PubMed]


