
Intravoxel incoherent motion diffusion-weighted MRI for the evaluation of early spleen involvement in acute leukemia
Introduction
Leukemia is considered a highly efficient metastatic cancer (1). Up to 80% of patients with acute leukemia (AL) had spleen involvement (2,3), characterized by increased cellularity with more blasts, and proliferation of endothelial cells with angiogenesis (1,4). Spleen involvement usually manifests as splenomegaly, which is associated with high tumor burden and poor prognosis in AL (5-7). Studies showed that the spleen can be a sanctuary site for residual disease after treatment and an important source for relapse (8,9). Some researchers have proposed that early splenectomy might be regarded as a promising adjunct to the treatment of AL (4,5,10). Therefore, the evaluation of spleen involvement, especially in the early stages, is important for individualized treatment as well as prognostic assessment.
Spleen biopsy is the gold standard for determining the presence and extent of leukemic infiltration. However, it is an invasive procedure that may lead to the spread of leukemia lesions and bleeding. Some imaging techniques [ultrasound, conventional computed tomography (CT)] are used to assess spleen involvement through splenic size, but they are difficult to find early involvement that causes no or only minimal increase in spleen volume (SV) (11). Semiquantitative contrast-enhanced CT can be applied to identify early spleen involvement in hematologic malignancies, indirectly reflecting cellular infiltration and microvascular changes (12-14). The standardized uptake value (SUV) from positron-emission tomography-computed tomography (PET-CT) is another functional imaging parameter used to evaluate early spleen involvement, which quantifies the number and metabolic activity of leukemia cells (15). Studies demonstrated that the spleen was the second highest organ for PET uptake after bone marrow in AL patients, and SUV values of the spleen in AL patients were significantly higher than those in controls (15,16).
Diffusion-weighted imaging (DWI), on the other hand, has been applied in assessing the spleen in hematologic malignancies, such as multiple myeloma and lymphomas, mainly based on the diffusion disorder caused by an increase in cell proliferation (17-19). Intravoxel incoherent motion (IVIM) is a DWI method that can simultaneously characterize diffusion and microcapillary perfusion in biological tissues (20). This method assumes a bi-exponential signal decay as a function of the b value, and relies on the acquisition of diffusion-weighted images with multiple b-values to extract a set of parameters: pure diffusion coefficient (D) that reflects tissue cellularity, pseudo-perfusion fraction (f) that represents the fraction of vascular volume, pseudo-diffusion coefficient (D*) that is related tissue microcapillary perfusion (21,22). Recently, attention has been paid to a new IVIM parameter, the perfusion-diffusion ratio (PDR), which may evaluate capillary permeability by calculating the relationship between the rate of S(b) signal decline induced by IVIM and that induced by diffusion (23,24). IVIM parameters have been utilized in characterizing solid and hematologic tumors (21,22,25,26). A study also confirmed that IVIM parameters were reliable imaging biomarkers for splenic changes in pancreatitis (27). We hypothesis that IVIM could be used to assess pathologic changes in the spleen from cellularity and angiogenesis and is a potential method for assessing early spleen involvement in AL patients.
In this preliminary study, we sought to use IVIM parameters to analyze functional abnormalities of the spleen based on cellularity and blood perfusion in patients with AL, expecting to identify early involvement of the spleen with normal volume. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-856/rc).
Methods
Study participants
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This prospective study was approved by the Ethics Committee of Second Hospital of Shanxi Medical University (No. 2020YX037), and all participants provided written informed consent. Between June 2020 and November 2022, patients with newly diagnosed AL as determined by the World Health Organization classification of hematopoietic tissue (28,29) were enrolled consecutively in the study. Inclusion criteria were patients who had no known history of splenic disease, splenic surgery, nor other diseases which may lead to spleen involvement (including immune system diseases, chronic liver disease, and cirrhosis), patients who had not previously received any treatment, and were eligible for magnetic resonance imaging (MRI). Exclusion criterion was poor IVIM images quality. The characteristics and baseline clinical biomarkers of patients with AL [e.g., age, sex, peripheral white blood cell (WBC) counts, lactate dehydrogenase (LDH), and bone marrow blasts] were collected. All enrolled patients were divided into splenomegaly group and normal SV group according to the personalized reference intervals for SV described in the “Definition of splenomegaly” section (30).
Healthy volunteers with no known history of malignant, hematological, splenic, hepatic, nor autoimmune disease were recruited as the control group, and those with splenomegaly according to the personalized reference intervals for SV described in the “Definition of splenomegaly” section were excluded (30).
MRI acquisition
All participants underwent abdominal IVIM DWI in the supine position with a 3.0 T MRI scanner (Discovery 750 w, GE Healthcare, Waukesha, WI, USA). IVIM DWI was performed using a respiratory-triggered single-shot spin echo-planar imaging pulse sequence, and a spectral spatial excitation pulse was used for fat suppression. The imaging parameters of IVIM DWI were: b=0, 10, 20, 30, 40, 50, 100 [number of excitations (NEX) =1], 200 (NEX =2), 400 (NEX =3), 800 sec/mm2 (NEX =4), repetition time =6,000 to 10,000 ms depending on the number of slices to adequately cover the anatomy, echo time =69.7 ms, slice thickness =6.0 mm, slice spacing =2.0 mm, field of view =40 cm × 40 cm, matrix =128×128. The acquisition time was approximately 4 min, depending upon the breathing.
Imaging analysis
All images were measured independently by two board-certified abdominal radiologists (with 7 and 3 years of abdominal imaging experience, respectively) who were blinded to clinical information.
Measurement of IVIM parameters
All IVIM images were processed on the workstation (Advantage Windows Workstation 4.6; GE Healthcare) to produce the parameters (D, D* and f). To reduce the effect of noise and overcome the mathematical instability of the IVIM model, a typical segmented fitting method was used for IVIM calculation, and the threshold b value was selected as 200 s/mm2 (27,31,32). Diffusion-weighted signal decay was analyzed according to the IVIM bi-exponential equation:
where Sb is the signal intensity at a given b value and S0 is the signal intensity for b=0 sec/mm2. First, D is estimated by mono-exponential fitting of the diffusion-weighted signals at high b values (b>200 s/mm2) assuming that perfusion contributions are negligible, according to the equation:
where Sint is the b=0 intercept of the mono-exponential fit of high b value data. f can be estimated according to Eq. [3]:
Finally, with the D and f, D* was calculated by fitting all data to Eq. [1].
Regions of interest (ROIs) were manually delineated on each splenic slice of the b=0 s/mm2 images by tracing the outline of spleens with the freehand ROI tool. Large vessels as well as areas with gross artifacts were avoided. The ROIs were copied to the D, D*, and f maps automatically, and the average parameter values and the number of voxels for each ROI were documented. Data for each spleen was expressed as the weighted average of IVIM parameters across all splenic sections. The PDR was calculated as (23,24):
Measurement of SV
SVs were measured using ITK-SNAP software (version 3.8.0, www.itksnap.org). The spleens were segmented manually by outlining each section of spleens on the b=0 s/mm2 images and the volumes were calculated automatically. This method has been validated in previous studies and shown optimal reproducibility (6,33).
Definition of splenomegaly
Individualized reference intervals for SV based on sex, age and anthropometric parameters were calculated using the web calculator (https://i-pacs.com/calculators) proposed by Kim et al. (30). Splenomegaly was defined as SV above the upper limit of the individualized reference intervals.
Statistical analysis
Interobserver agreements for SV and IVIM parameters were evaluated by calculating the interclass correlation coefficient (ICC). Categorical data were tested using the Chi-squared test. One-way analysis of variance was used for the comparisons of the clinical and MRI parameters among the three groups. Post hoc multiple pairwise comparisons were done using the LSD or Tamhane’s T2 tests. Area under the curve (AUC) of the receiver operating characteristic (ROC) analysis was used to evaluate the diagnostic efficacy of IVIM parameters. The optimal cutoff values were determined by the Youden index. DeLong test was applied to compare the AUC values between the different parameters. Correlation analyses were performed using Spearman correlation. Statistical analyses were conducted using SPSS statistical software (version 26.0, IBM, Armonk, NY, USA) and MedCalc statistical software (version 20.0.22). P<0.05 (two-sided test) was considered statistically significant.
Results
Study participants and clinical characteristics
Eighty-two patients with AL and 57 healthy volunteers underwent IVIM DWI in the abdomen. Figure 1 shows the participant selection flowchart. Ultimately, 79 patients with AL (AL with splenomegaly: n=54, 68%; AL with normal SV: n=25, 32%) and 55 healthy volunteers (control group) were enrolled in this study (Table 1). There were no significant differences in age and sex among the three groups (P=0.649 and P=0.462, respectively). The WBC counts and LDH of the AL patients with splenomegaly were significantly higher than those of the AL with normal SV (P=0.042 and P=0.006, respectively). There was no significant difference in bone marrow blasts between the subgroups of AL (P=0.639) (Table 1).

Table 1
| Variable | AL with splenomegaly (n=54) | AL with normal SV (n=25) | Control group (n=55) | P value |
|---|---|---|---|---|
| Sex | 0.462† | |||
| Male | 27 (50.0) | 14 (56.0) | 34 (61.8) | |
| Female | 27 (50.0) | 11 (44.0) | 21 (38.2) | |
| Age (years) | 45±18 | 42±18 | 44±18 | 0.649‡ |
| SV (cm × cm × cm) | 448.6 (345.7–674.1) | 198.2 (167.6–257.6) | 177.8 (144.5–230.4) | <0.001§ |
| WBC counts (×109) | 15.9 (4.2–54.4) | 5.5 (2.5–17.8) | 0.042¶ | |
| LDH (U/L) | 524.6 (290.5–869.8) | 270.0 (210.0–402.0) | 0.006¶ | |
| Bone marrow blasts (%) | 72.4 (45.4–86.8) | 62.4 (43.4–78.8) | 0.639¶ |
Data are shown as n (%), mean ± standard deviation, and median (interquartile range). †, determined with the χ2 test. ‡, determined with the one-way ANOVA test. §, determined with the Kruskal-Wallis test. ¶, determined with the Mann-Whitney U test. AL, acute leukemia; SV, spleen volume; WBC, white blood cell; LDH, lactate dehydrogenase.
Inter-reader variability
The ICC values of IVIM parameters were 0.91 (95% CI: 0.86–0.94) for D, 0.72 (95% CI: 0.62–0.84) for D*, 0.84 (95% CI: 0.76–0.92) for f (all P<0.001), and the ICC value of SV was 0.95 (95% CI: 0.93–0.98, P<0.001), indicating good or excellent agreement. As a consequence, only the results from the first radiologist were analyzed in our study.
Differences of IVIM parameters among controls, AL patients with splenomegaly and AL patients with normal SV
The representative IVIM images of the three groups are shown in Figure 2. Comparisons of IVIM parameters among the three groups are presented in Table 2 and Figure 3. D showed statistically significant differences among the controls, AL patients with normal SV, and AL patients with splenomegaly [(0.99±0.19)×10−3vs. (0.78±0.16)×10−3 vs. (0.66±0.11)×10−3 mm2/s, P<0.001]. D* value in the AL with splenomegaly group was significantly lower than that in the AL with normal SV group and the control group (P=0.004 and P=0.001, respectively), while f value was significantly higher than that in the AL with normal SV and the control groups (P=0.046 and P<0.001, respectively). D* and f values had no significant difference between the AL with normal SV and the control groups (P=0.913, and P=0.195, respectively). AL with splenomegaly and normal SV groups showed statistically higher PDR values than the control group (P<0.001, and P=0.013, respectively). However, there was no significant difference in PDR between AL with splenomegaly and normal SV groups (P=0.451).
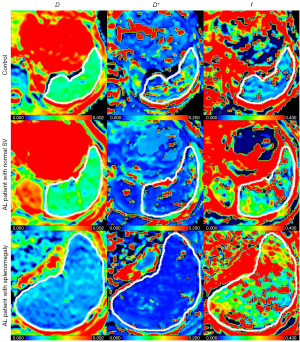
Table 2
| Parameter | AL with splenomegaly (n=54) | AL with normal SV (n=25) | Control group (n=55) | P value | Pa | Pb | Pc |
|---|---|---|---|---|---|---|---|
| D (×10−3 mm2/s)† | 0.66±0.11 | 0.78±0.16 | 0.99±0.19 | <0.001 | <0.001 | 0.002 | <0.001 |
| D* (×10−3 mm2/s)‡ | 114.7±34.6 | 146.5±40.4 | 153.0±55.1 | <0.001 | 0.001 | 0.004 | 0.913 |
| f (%)‡ | 27.4±7.7 | 24.7±5.0 | 22.5±4.8 | <0.001 | <0.001 | 0.046 | 0.195 |
| PDR† | 68.3±33.5 | 63.1±22.6 | 47.9±25.9 | 0.001 | <0.001 | 0.451 | 0.031 |
Data are shown as mean ± standard deviation. IVIM parameters were compared by one-way ANOVA. †, data are compared by using LSD post hoc test; ‡, data are compared by using Tamhane’s T2 post hoc test; a, post hoc paired comparisons between AL with splenomegaly group and the control group; b, post hoc paired comparisons between AL with splenomegaly group and AL with normal SV group; c, post hoc paired comparisons between AL with normal SV group and the control group. IVIM, intravoxel incoherent motion; AL, acute leukemia; SV, spleen volume; D, diffusion coefficient; D*, pseudo-diffusion coefficient; f, pseudo-perfusion fraction; PDR, perfusion-diffusion ratio.

Correlations of MRI parameters with clinical biomarkers
D value of the spleen was negatively correlated with WBC counts (r=−0.424; 95% CI: −0.570, −0.211; P<0.001), LDH (r=−0.285; 95% CI: −0.486, −0.011; P=0.011), and bone marrow blasts (r=−0.283; 95% CI: −0.476, −0.067; P=0.012). D* value was negatively correlated with LDH (r=−0.276; 95% CI: −0.470, −0.025; P=0.014), while f and PDR showed positive correlation with LDH (r=0.514; 95% CI: 0.342, 0.664; P<0.001 and r=0.343; 95% CI: 0.208, 0.549; P=0.002, respectively) (Table 3).
Table 3
| Parameters | WBC counts | LDH | Bone marrow blasts | |||||
|---|---|---|---|---|---|---|---|---|
| r (95% CI) | P | r (95% CI) | P | r (95% CI) | P | |||
| D | −0.424 (−0.570, −0.211) | <0.001 | −0.285 (−0.486, −0.011) | 0.011 | −0.283 (−0.476, −0.067) | 0.012 | ||
| D* | −0.002 (−0.203, 0.212) | 0.986 | −0.276 (−0.470, −0.025) | 0.014 | −0.072 (−0.259, 0.143) | 0.530 | ||
| f | 0.028 (−0.185, 0.240) | 0.808 | 0.514 (0.342, 0.664) | <0.001 | 0.019 (−0.192, 0.235) | 0.868 | ||
| PDR | 0.156 (−0.071, 0.366) | 0.170 | 0.343 (0.208, 0.549) | 0.002 | 0.119 (−0.110, 0.316) | 0.297 | ||
IVIM, intravoxel incoherent motion; AL, acute leukemia; WBC, white blood cell; LDH, lactate dehydrogenase; D, diffusion coefficient; D*, pseudo-diffusion coefficient; f, pseudo-perfusion fraction; PDR, perfusion-diffusion ratio.
Diagnostic performance of IVIM parameters
Figure 4 depicts the ROC curves of IVIM parameters for evaluating functional changes of the spleen in AL patients. The corresponding diagnostic characteristics are shown in Table 4. The combination of D, f, and D* (AUC: 0.987; 95% CI: 0.944, 0.999) demonstrated better diagnostic efficacy than the single indicator D (AUC: 0.969; 95% CI: 0.917, 0.993; Delong test: Z=2.032, P=0.042), D* (AUC: 0.714; 95% CI: 0.620, 0.797; Delong test: Z=5.524, P<0.001), f (AUC: 0.733; 95% CI: 0.639, 0.813; Delong test: Z=5.246, P<0.001), and PDR (AUC: 0.692; 95% CI: 0.596, 0.777; Delong test: Z=5.860, P<0.001) in differentiation between the splenomegaly subgroup and the control group. The AUC of D combined with f and D* (AUC: 0.830; 95% CI: 0.729, 0.905) was higher than the single indicator D* (AUC: 0.721; 95% CI: 0.608, 0.816; Delong test: Z=2.012, P=0.044) and f (AUC: 0.647; 95% CI: 0.532, 0.752; Delong test: Z=2.829, P=0.005), but was not significantly different from single D (AUC: 0.756; 95% CI: 0.647, 0.846; Delong test: Z=1.676, P=0.094) in differentiating the splenomegaly group and the normal SV group. There was no significant difference in the AUC between D (AUC: 0.804; 95% CI: 0.700, 0.884) and PDR (AUC: 0.692; 95% CI: 0.579, 0.791) for comparing AL with normal SV group and the control group (Z=1.746, P=0.081).

Table 4
| Parameters | AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | P value |
|---|---|---|---|---|---|
| AL with splenomegaly (n=54) vs. control group (n=55) | |||||
| D (10−3 mm2/s) | 0.969 (0.917, 0.993) | 0.78 | 92.59 | 92.73 | <0.001 |
| D* (10−3 mm2/s) | 0.714 (0.620, 0.797) | 163.1 | 92.59 | 47.27 | <0.001 |
| f (%) | 0.733 (0.639, 0.813) | 26.2 | 62.96 | 78.18 | <0.001 |
| PDR | 0.692 (0.596, 0.777) | 42.2 | 83.33 | 52.73 | <0.001 |
| D+D*+ f | 0.987 (0.944, 0.999) | 0.60 | 92.59 | 100 | <0.001 |
| AL with splenomegaly (n=54) vs. AL with normal SV (n=25) | |||||
| D (10−3 mm2/s) | 0.756 (0.647, 0.846) | 0.78 | 90.74 | 56.00 | <0.001 |
| D* (10−3 mm2/s) | 0.721 (0.608, 0.816) | 113.3 | 53.70 | 84.00 | <0.001 |
| f (%) | 0.647 (0.532, 0.752) | 29.0 | 42.59 | 92.00 | 0.017 |
| D+D*+ f | 0.830 (0.729, 0.905) | 0.76 | 68.52 | 88.00 | <0.001 |
| AL with normal SV (n=25) vs. control group (n=55) | |||||
| D (10−3 mm2/s) | 0.804 (0.700, 0.884) | 0.75 | 56.00 | 96.36 | <0.001 |
| PDR | 0.692 (0.579, 0.791) | 40.5 | 88.00 | 49.09 | 0.001 |
IVIM, intravoxel incoherent motion; AUC, area under the curve; CI, confidence intervals; AL, acute leukemia; D, diffusion coefficient; D*, pseudo-diffusion coefficient; f, pseudo-perfusion fraction; PDR, perfusion-diffusion ratio; SV, spleen volume.
Discussion
The spleen is a frequent organ of leukemia metastasis (1). It is important to detect early spleen involvement that has not yet caused splenomegaly. Our results indicated that there were significant differences in IVIM parameters among the controls, AL patients with normal SV, and AL patients with splenomegaly, and that D and PDR may identify early spleen involvement in patients with AL.
Spleen involvement has been reported in up to 80% of patients with AL (2,3). Splenomegaly is an important clinical manifestation of spleen infiltration by leukemia (5-7). Compared to previous studies using splenic palpation or cross-sectional imaging to determine splenomegaly (7,13), our study identified splenomegaly more precisely according to the personalized reference interval for three-dimensional SV proposed by Kim et al. (30), and the result showed that 68% of AL patients had splenomegaly. However, morphological changes of the spleen are often not observed in the early stages of spleen involvement. The pathological changes of spleen involvement commonly include: (I) increased cellularity with more blasts entering the spleen through large and fenestrated sinusoidal vessels in the red pulp (1); and (II) pathological angiogenesis of the spleen, which can promote the rapid proliferation of malignant blasts in the spleen and the progression of the disease (4,5). Previous studies demonstrated that IVIM parameters can be utilized in characterizing the cellularity and angiogenesis of bone marrow and renal parenchyma in AL patients (22,25,26,34). In the present study, the parameters from IVIM showed significant differences among the control group, AL with normal SV group, and AL with splenomegaly group, suggesting they could be used to analyze functional changes of the spleen in patients with AL.
In our study, D value of the spleen decreased successively among the three groups, which may reflect the diffusion alterations caused by hypercellularity. DWI based on apparent diffusion coefficient (ADC) value has been applied in assessing the spleen in hematologic malignancies, such as multiple myeloma and lymphomas (17-19). Compared with conventional ADC acquired from DWI, D was the pure diffusion coefficient excluding the effect of microcirculation, which can more precisely reflect water diffusion in tissues. Our results showed that D value exhibited good diagnostic performance with an AUC of 0.804 in the differentiation between the control and AL with normal SV groups, indicating that D is a useful MRI biomarker for identifying early pathologic changes in the spleen of AL patients. Additionally, D value of the spleen was negatively correlated with WBC counts and bone marrow blasts, further confirming that a higher D value in AL may be due to the hypercellularity caused by leukemic blasts entering the spleen.
Compared with the controls and AL patients with normal SV, D* value was decreased, and f value was increased in AL patients with splenomegaly, which may reflect the pathological changes in the splenic vessels of AL. The parameters of contrast-enhanced CT and dynamic contrast-enhanced MRI have been used to access splenic perfusion and infiltration of hematologic malignancies in several studies (12,13,35). The use of D* reflecting blood flow velocity and f representing vascular volume fraction from IVIM provides an alternative means to estimate hemodynamic characteristics without using a contrast agent, facilitating adoption in clinical practice (22). Shaked et al. reported that the stained vessel area and vascular density in the spleen of AL mice were significantly higher than those of normal control mice (4). Proangiogenic response mechanism in the leukemic spleen can lead to the up-regulation of key angiogenic factors as well as the activation and proliferation of endothelial cells, resulting in pathological angiogenesis (4,5), which could be reflected by the increased f value (34,36). In the progression of AL, more leukemic cells are recruited to the spleen, leading to leukostasis, vessel clogging, and slowing of blood flow velocity (37,38), which may account for the decrease in D* value. In addition, D* and f values are correlated with LDH, a tumor burden indicator that can promote tumor cell metastasis and tumor angiogenesis (39), contributing to further understanding the pathophysiological significance of D* and f. However, no significant differences in the D* and f values were found between the AL with normal SV group and the control group, probably because splenic angiogenesis and leukostasis were not obvious before splenomegaly.
PDR is another parameter of IVIM that expresses the ratio of water flow within and without the capillary network (23). It not only characterizes the fast component of DWI but also depicts the relationship between diffusion and perfusion. PDR combines all IVIM parameters in one, making it more sensitive for evaluating the pathological changes of the tissue. It has been used to differentiate liver space-occupying lesions and evaluate changes of brain parenchyma (23,24). In our study, PDR showed good discrimination with a sensitivity of 88% in the comparison between the control and patients with normal SV groups, suggesting that PDR could be a useful parameter in assessing early spleen involvement. However, it is worth noting that PDR may be affected by the high variability of D* values (40,41), so its reliability and clinical utility need to be confirmed by further studies. There was no significant difference between the two AL subgroups, probably because both perfusion and diffusion changed during the development of splenomegaly, resulting in no significant difference in their ratio.
Our result indicated that the combination of D, f, and D* demonstrated better diagnostic efficacy than the single D* and f in differentiating AL with normal SV group and splenomegaly group. However, the combined diagnosis was not significantly superior to the single D. Therefore, a single indicator D is more practical for clinical application.
D values of the spleen from healthy controls in our study were similar to those reported previously (42-47). However, f values were higher than those in some studies, although they were still within the range reported in the literature (f ranges from 0.076%±0.007% to 0.31%±0.13%) (42-47), which may be related to the differences in the ROI delineation, MR imager (43), acquisition mechanism (44), b value distribution (48), and fitting method (45).
There were several limitations in the current study. First, IVIM modeling of the perfusion component could be constrained by the diffusion component as reported (49), so the higher f value may be affected by the lower D value. Further technical improvements for IVIM modeling are needed to strengthen the robustness of IVIM parameters. Second, the association of IVIM parameters and histologic features would aid in validating the pathophysiologic meanings of the splenic IVIM parameters. Third, although spleen infiltration is expected in most subjects, those with a normal sized spleen who did not present with spleen infiltration cannot be completely excluded, and changes in splenic IVIM parameters after treatment may help to determine spleen infiltration. Finally, the sample size was small, and further studies need to be performed to explore the differences in acute lymphoblastic leukemia and acute myelocytic leukemia.
In conclusion, IVIM diffusion-weighted MRI could be a potential alternative for assessing pathologic changes in the spleen from cellularity and angiogenesis, and D and PDR may be viable indicators to identify early spleen involvement in patients with AL.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-856/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-856/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Whiteley AE, Price TT, Cantelli G, Sipkins DA. Leukaemia: a model metastatic disease. Nat Rev Cancer 2021;21:461-75. [Crossref] [PubMed]
- Viadana E, Bross ID, Pickren JW. An autopsy study of the metastatic patterns of human leukemias. Oncology 1978;35:87-96. [Crossref] [PubMed]
- Barcos M, Lane W, Gomez GA, Han T, Freeman A, Preisler H, Henderson E. An autopsy study of 1206 acute and chronic leukemias (1958 to 1982). Cancer 1987;60:827-37. [Crossref] [PubMed]
- Shaked Y, Cervi D, Neuman M, Chen L, Klement G, Michaud CR, Haeri M, Pak BJ, Kerbel RS, Ben-David Y. The splenic microenvironment is a source of proangiogenesis/inflammatory mediators accelerating the expansion of murine erythroleukemic cells. Blood 2005;105:4500-7. [Crossref] [PubMed]
- Ma S, Shi Y, Pang Y, Dong F, Cheng H, Hao S, Xu J, Zhu X, Yuan W, Cheng T, Zheng G. Notch1-induced T cell leukemia can be potentiated by microenvironmental cues in the spleen. J Hematol Oncol 2014;7:71. [Crossref] [PubMed]
- Shimomura Y, Hara M, Katoh D, Hashimoto H, Ishikawa T. Enlarged spleen is associated with low neutrophil and platelet engraftment rates and poor survival after allogeneic stem cell transplantation in patients with acute myeloid leukemia and myelodysplastic syndrome. Ann Hematol 2018;97:1049-56. [Crossref] [PubMed]
- Shuster JJ, Falletta JM, Pullen DJ, Crist WM, Humphrey GB, Dowell BL, Wharam MD, Borowitz M. Prognostic factors in childhood T-cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood 1990;75:166-73.
- Di Grande A, Peirs S, Donovan PD, Van Trimpont M, Morscio J, Lintermans B, Reunes L, Vandamme N, Goossens S, Nguyen HA, Lavie A, Lock RB, Prehn JHM, Van Vlierberghe P, Ní Chonghaile T. The spleen as a sanctuary site for residual leukemic cells following ABT-199 monotherapy in ETP-ALL. Blood Adv 2021;5:1963-76. [Crossref] [PubMed]
- Cheng H, Sun G, Cheng T. Hematopoiesis and microenvironment in hematological malignancies. Cell Regen 2018;7:22-6. [Crossref] [PubMed]
- Fleming I, Simone J, Jackson R, Johnson W, Walters T, Mason C. Cancer 1974;33:427-34. [Crossref] [PubMed]
- Saboo SS, Krajewski KM, O'Regan KN, Giardino A, Brown JR, Ramaiya N, Jagannathan JP. Spleen in haematological malignancies: spectrum of imaging findings. Br J Radiol 2012;85:81-92. [Crossref] [PubMed]
- Reinert CP, Hinterleitner C, Fritz J, Nikolaou K, Horger M. Diagnosis of diffuse spleen involvement in haematological malignancies using a spleen-to-liver attenuation ratio on contrast-enhanced CT images. Eur Radiol 2019;29:450-7. [Crossref] [PubMed]
- Reinert CP, Kloth C, Fritz J, Nikolaou K, Horger M. Discriminatory CT-textural features in splenic infiltration of lymphoma versus splenomegaly in liver cirrhosis versus normal spleens in controls and evaluation of their role for longitudinal lymphoma monitoring. Eur J Radiol 2018;104:129-35. [Crossref] [PubMed]
- Sauter AW, Feldmann S, Spira D, Schulze M, Klotz E, Vogel W, Claussen CD, Horger MS. Assessment of splenic perfusion in patients with malignant hematologic diseases and spleen involvement, liver cirrhosis and controls using volume perfusion CT (VPCT): a pilot study. Acad Radiol 2012;19:579-87. [Crossref] [PubMed]
- Zhou WL, Wu HB, Wang LJ, Tian Y, Dong Y, Wang QS. Usefulness and pitfalls of F-18-FDG PET/CT for diagnosing extramedullary acute leukemia. Eur J Radiol 2016;85:205-10. [Crossref] [PubMed]
- Buck AK, Bommer M, Juweid ME, Glatting G, Stilgenbauer S, Mottaghy FM, Schulz M, Kull T, Bunjes D, Möller P, Döhner H, Reske SN. First demonstration of leukemia imaging with the proliferation marker 18F-fluorodeoxythymidine. J Nucl Med 2008;49:1756-62. [Crossref] [PubMed]
- Rasche L, Kumar M, Gershner G, Samant R, Van Hemert R, Heidemeier A, Lapa C, Bley T, Buck A, McDonald J, Hillengass J, Epstein J, Thanendrarajan S, Schinke C, van Rhee F, Zangari M, Barlogie B, Davies FE, Morgan GJ, Weinhold N. Lack of Spleen Signal on Diffusion Weighted MRI is associated with High Tumor Burden and Poor Prognosis in Multiple Myeloma: A Link to Extramedullary Hematopoiesis? Theranostics 2019;9:4756-63. [Crossref] [PubMed]
- Littooij AS, Kwee TC, Barber I, Granata C, de Keizer B, Beek FJ, Hobbelink MG, Fijnheer R, Stoker J, Nievelstein RA. Accuracy of whole-body MRI in the assessment of splenic involvement in lymphoma. Acta Radiol 2016;57:142-51. [Crossref] [PubMed]
- Kharuzhyk S, Zhavrid E, Dziuban A, Sukolinskaja E, Kalenik O. Comparison of whole-body MRI with diffusion-weighted imaging and PET/CT in lymphoma staging. Eur Radiol 2020;30:3915-23. [Crossref] [PubMed]
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497-505. [Crossref] [PubMed]
- Tang L, Zhou XJ. Diffusion MRI of cancer: From low to high b-values. J Magn Reson Imaging 2019;49:23-40. [Crossref] [PubMed]
- Li J, Li W, Niu J, Song X, Wu W, Gong T, Zheng R, Ting-Fang Shih T, Li W, Zhou XJ. Intravoxel Incoherent Motion Diffusion-weighted MRI of Infiltrated Marrow for Predicting Overall Survival in Newly Diagnosed Acute Myeloid Leukemia. Radiology 2020;295:155-61. [Crossref] [PubMed]
- Podgórska J, Pasicz K, Skrzyński W, Gołębiewski B, Kuś P, Jasieniak J, Kiliszczyk A, Rogowska A, Benkert T, Pałucki J, Grabska I, Fabiszewska E, Jagielska B, Kukołowicz P, Cieszanowski A. Perfusion-Diffusion Ratio: A New IVIM Approach in Differentiating Solid Benign and Malignant Primary Lesions of the Liver. Biomed Res Int 2022;2022:2957759. [Crossref] [PubMed]
- Cui J, Zheng J, Niu W, Bian W, Wang J, Niu J. Quantitative IVIM parameters evaluating perfusion changes in brain parenchyma in patients newly diagnosed with acute leukemia: Compared with healthy participants. Front Neurol 2023;14:1093003. [Crossref] [PubMed]
- Niu J, Li W, Wang H, Wu W, Gong T, Huang N, Wang J, Qi Y. Intravoxel incoherent motion diffusion-weighted imaging of bone marrow in patients with acute myeloid leukemia: a pilot study of prognostic value. J Magn Reson Imaging 2017;46:476-82. [Crossref] [PubMed]
- Li J, Liu S, Bian W, Yuan Z, Wang J, Niu J. Intravoxel incoherent motion diffusion-weighted MRI of renal parenchyma and its clinical significance in patients with untreated acute leukemia: a pilot study. Abdom Radiol (NY) 2023;48:1363-71. [Crossref] [PubMed]
- Xie CL, Zhang M, Chen Y, Hu R, Tang MY, Chen TW, Xue HD, Jin ZY, Zhang XM. Spleen and splenic vascular involvement in acute pancreatitis: an MRI study. Quant Imaging Med Surg 2018;8:291-300. [Crossref] [PubMed]
- Morphologic, immunologic, and cytogenetic (MIC) working classification of acute lymphoblastic leukemias. Report of the workshop held in Leuven, Belgium, April 22-23, 1985. First MIC Cooperative Study Group. Cancer Genet Cytogenet 1986;23:189-97.
- Morphologic, immunologic, and cytogenetic (MIC) working classification of the acute myeloid leukemias. Report of the Workshop held in Leuven, Belgium, September 15-17, 1986. Second MIC Cooperative Study Group. Cancer Genet Cytogenet 1988;30:1-15.
- Kim DW, Ha J, Lee SS, Kwon JH, Kim NY, Sung YS, Yoon JS, Suk HI, Lee Y, Kang BK. Population-based and Personalized Reference Intervals for Liver and Spleen Volumes in Healthy Individuals and Those with Viral Hepatitis. Radiology 2021;301:339-47. [Crossref] [PubMed]
- Park HJ, Sung YS, Lee SS, Lee Y, Cheong H, Kim YJ, Lee MG. Intravoxel incoherent motion diffusion-weighted MRI of the abdomen: The effect of fitting algorithms on the accuracy and reliability of the parameters. J Magn Reson Imaging 2017;45:1637-47. [Crossref] [PubMed]
- Zhang Y, Kuang S, Shan Q, Rong D, Zhang Z, Yang H, Wu J, Chen J, He B, Deng Y, Roberts N, Shen J, Venkatesh SK, Wang J. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol 2019;29:5791-803. [Crossref] [PubMed]
- Son JH, Lee SS, Lee Y, Kang BK, Sung YS, Jo S, Yu E. Assessment of liver fibrosis severity using computed tomography-based liver and spleen volumetric indices in patients with chronic liver disease. Eur Radiol 2020;30:3486-96. [Crossref] [PubMed]
- Li J, Zheng R, Niu J, Song X, Wu W, Fan R, Gong T. Correlation of Intravoxel Incoherent Motion Parameters and Histological Characteristics From Infiltrated Marrow in Patients With Acute Leukemia. J Magn Reson Imaging 2020;51:1720-6. [Crossref] [PubMed]
- Punwani S, Cheung KK, Skipper N, Bell N, Bainbridge A, Taylor SA, Groves AM, Hain SF, Ben-Haim S, Shankar A, Daw S, Halligan S, Humphries PD. Dynamic contrast-enhanced MRI improves accuracy for detecting focal splenic involvement in children and adolescents with Hodgkin disease. Pediatr Radiol 2013;43:941-9. [Crossref] [PubMed]
- Fan R, Zhu H, Niu J, Li J, Zheng R, Song X. Correlation of histological marrow characteristics and intravoxel incoherent motion-derived parameters in benign and malignant hematological disorders. Eur J Radiol 2020;123:108745. [Crossref] [PubMed]
- De Santis GC, Oliveira LC, Ramos AF, da Silva ND, Falcão RP. Pathologic rupture of the spleen in a patient with acute myelogenous leukemia and leukostasis. Rev Bras Hematol Hemoter 2014;36:290-2. [Crossref] [PubMed]
- Tan A, Ziari M, Salman H, Ortega W, Cortese C. Spontaneous rupture of the spleen in the presentation of acute myeloid leukemia. J Clin Oncol 2007;25:5519-20. [Crossref] [PubMed]
- Feng Y, Xiong Y, Qiao T, Li X, Jia L, Han Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med 2018;7:6124-36. [Crossref] [PubMed]
- Chevallier O, Zhou N, Cercueil JP, He J, Loffroy R, Wáng YXJ. Comparison of tri-exponential decay versus bi-exponential decay and full fitting versus segmented fitting for modeling liver intravoxel incoherent motion diffusion MRI. NMR Biomed 2019;32:e4155. [Crossref] [PubMed]
- Lee Y, Lee SS, Kim N, Kim E, Kim YJ, Yun SC, Kühn B, Kim IS, Park SH, Kim SY, Lee MG. Intravoxel incoherent motion diffusion-weighted MR imaging of the liver: effect of triggering methods on regional variability and measurement repeatability of quantitative parameters. Radiology 2015;274:405-15. [Crossref] [PubMed]
- Yu WL, Xiao BH, Ma FZ, Zheng CJ, Tang SN, Wáng YXJ. Underestimation of the spleen perfusion fraction by intravoxel incoherent motion MRI. NMR Biomed 2023;36:e4987. [Crossref] [PubMed]
- Barbieri S, Donati OF, Froehlich JM, Thoeny HC. Comparison of Intravoxel Incoherent Motion Parameters across MR Imagers and Field Strengths: Evaluation in Upper Abdominal Organs. Radiology 2016;279:784-94. [Crossref] [PubMed]
- Jerome NP, Orton MR, d'Arcy JA, Collins DJ, Koh DM, Leach MO. Comparison of free-breathing with navigator-controlled acquisition regimes in abdominal diffusion-weighted magnetic resonance images: Effect on ADC and IVIM statistics. J Magn Reson Imaging 2014;39:235-40. [Crossref] [PubMed]
- Barbieri S, Donati OF, Froehlich JM, Thoeny HC. Impact of the calculation algorithm on biexponential fitting of diffusion-weighted MRI in upper abdominal organs. Magn Reson Med 2016;75:2175-84. [Crossref] [PubMed]
- Taimouri V, Afacan O, Perez-Rossello JM, Callahan MJ, Mulkern RV, Warfield SK, Freiman M. Spatially constrained incoherent motion method improves diffusion-weighted MRI signal decay analysis in the liver and spleen. Med Phys 2015;42:1895-903. [Crossref] [PubMed]
- Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 1999;210:617-23. [Crossref] [PubMed]
- Lemke A, Stieltjes B, Schad LR, Laun FB. Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging 2011;29:766-76. [Crossref] [PubMed]
- Wáng YXJ. Mutual constraining of slow component and fast component measures: some observations in liver IVIM imaging. Quant Imaging Med Surg 2021;11:2879-87. [Crossref] [PubMed]


