
Noninvasive carotid ultrasound for predicting vulnerable plaques of the coronary artery based on optical coherence tomography images
Introduction
Atherosclerosis is a systemic and complicated pathological change that can occur simultaneously in a multi-vessel bed. Atherosclerotic thromboembolism is closely related to plaque irregularity and rupture. Autopsy studies have shown that the main pathological characteristics of vulnerable plaques were a large lipid necrotic core, a thin fibrous cap, and macrophage infiltration; lipid plaques are shown as hypoechoic plaques on carotid ultrasound imaging. On optical coherence tomography (OCT) examination, vulnerable plaques are defined as the areas with blurred edges, high back reflection, and strong attenuation, and a fibrous cap can be seen with a high signal band on the surface of the low signal region. Atherosclerosis of coronary arteries can cause serious cardiovascular events and is one of the important causes of death worldwide. Due to the superficial position of the carotid artery and its proximity to the coronary artery, many studies have demonstrated that carotid intima-media thickness, plaque load, and calcification can predict coronary artery events, but controversy remains (1-4). Rupture of vulnerable coronary plaque is the main cause of sudden obstructive myocardial infarction in patients previously recorded as having less than 50% coronary stenosis (5). However, the use of noninvasive methods to predict the risk of coronary vulnerable plaques is rarely reported. The irregularity and rupture of vulnerable plaques tend to occur systematically, and the risk and vulnerability of coronary and carotid plaque formation are similar (5-12); plaque composition and fibrous cap level are strongly correlated with carotid and coronary events. Carotid ultrasound can observe vulnerable plaque, that is, plaque with high lipid content in the low echo area. Coronary interventional imaging OCT can identify vulnerable plaques with high lipid content and thin fibrous caps (12).
The purpose of this study was to predict the vulnerability of coronary artery plaque in patients with stable angina pectoris (SAP) through the characteristics of carotid artery plaque, and provide a new method for the diagnosis of coronary heart disease. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-621/rc).
Methods
Participants
This was a retrospective case-control study during 2016 to 2021. Patients with SAP of coronary artery disease who completed OCT examination during percutaneous coronary intervention (PCI) surgery and carotid ultrasound examination within 3 days before the PCI surgery in Beijing Anzhen Hospital were enrolled in this study.
The inclusion criteria were as follows: (I) patients <75 years old; (II) patients with SAP who had at least one major coronary artery branch (anterior descending, right coronary, and circumflex branches) examined by OCT; (III) patients who underwent coronary revascularization PCI or coronary artery bypass grafting (CABG). The exclusion criteria were as follows: (I) patients with severe heart failure, abnormal liver and kidney function, and malignant tumor; (II) patients with severe left main coronary artery disease or chronic complete occlusive disease; (III) patients with incomplete clinical data, unclear OCT images, or insufficient number of coronary angiography positions, which affect the collection of case data.
According to the OCT diagnostic criteria for coronary artery vulnerable plaques, 35 patients with confirmed coronary vulnerable plaque were selected as the case group, and 35 patients with stable coronary plaque were randomly selected as the control group. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Anzhen Hospital (No. 2022147X). All patients provided informed consent. Carotid plaque characteristics were assessed separately by one author and one independent specialist in vascular ultrasound (not an author) blinded to the outcomes.
Data collection and definitions
The full medical records of patients were collected, including their clinical characteristics, laboratory results, cardiac and coronary artery imaging results, demographic data, medical and pharmacological history, and treatment strategies at discharge.
The body mass index (BMI) was expressed as the ratio of body weight in kilograms divided by height in square meters (kg/m2). According to the International Society of Hypertension guidelines (13), hypertension was defined as persistently elevated systolic blood pressure >140 mmHg and/or diastolic blood pressure ≥90 mmHg (without antihypertensive drugs). Dyslipidemia was defined according to criteria of the 2007 Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults (14), that refers to total cholesterol >5.2 mmol/L, fasting triglycerides >1.7 mmol/L, and low-density lipoprotein cholesterol >3.4 mmol/L. The criteria for diabetes mellitus were fasting plasma glucose ≥7.0 mmol/L, spontaneous glucose level ≥11.1 mmol/L, or glycosylated hemoglobin (HbA1c) >6.5% (15). Former smokers and patients who still smoked were classified as smokers. C-reactive protein (CRP) levels were determined using Quik Read CRP test (Orion Diagnostica, Espoo, Finland). Elevated CRP was defined as >5 mg/L.
Blood lipids and CRP were analyzed on an automatic biochemical analyzer (AU5400; Beckman Coulter, Brea, CA, USA).
Ultrasound examination method
A carotid artery ultrasound was performed within the 3 days before coronary angiography using a PREVI color Doppler ultrasound diagnostic instrument (Hitachi, Tokyo, Japan).
High-frequency linear array probes (5–13 MHz; 4–9 MHz) were used to acquire real-time images (axial and transverse gray-scale images as well as color blood flow images were stored). The distal end of the internal carotid artery and the initial section of the common carotid artery were examined with a fan-shaped probe at 2.5–5 MHz.
B-mode ultrasound settings
- Dynamic range: 55–80 dB (average, 60–70 dB).
- Scan depth: the scan depth was set at approximately 4 cm, and adjusted based on the depth of the anatomical location of the patient’s carotid artery. The deeper the anatomical position of the carotid artery, the greater the scan depth, and the carotid structure being clearly displayed by B-ultrasound was the setting criterion.
Doppler ultrasound settings
- Doppler ultrasound gain: the color gain was increased to the level at which the noise outside the blood vessel first appeared, and was then gradually reduced until the noise outside the blood vessel had almost disappeared.
- Doppler ultrasound velocity range: 20–50 cm/s. The flow range and baseline were appropriately adjusted based on the patient’s carotid flow velocity to avoid aliasing due to high carotid flow and low set flow level, and the inability to display low velocity interaction flow due to the patient’s low carotid flow and high set flow level.
- Doppler sampling frame: the sampling frame was located at the center of the lumen or narrow vascular bundle and was generally set at 2 mm; it could be appropriately adjusted to 1/2–1/3 of the lumen diameter depending on the internal diameter of the patient’s carotid artery.
- Angle correction: the correction angle was kept as small as possible, not exceeding 60°.
- Doppler filter: a low-frequency wall filter was used to remove the movement signal of the blood vessel wall and surrounding tissues, yet attention was also paid to the low-speed blood flow signal during diastole.
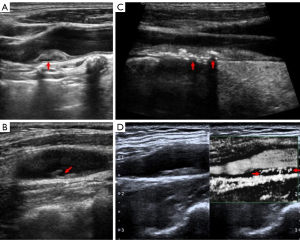
The following indicators were recorded: whether the carotid intima-media was thickened (≥1.0 mm), whether the plaque surface was regular, whether the plaque echo was homogeneous, whether the plaque was a low-echo plaque, whether the plaque was a mixed-echo plaque dominated with low echo, whether the plaque had calcification, the thickness of the plaque (<3.0 or ≥3.0 mm), whether there was carotid stenosis (diameter stenosis rate: ≥40%), and whether the plaque was a neovascular plaque (10). Plaque echo definition: Those consistent with sternocleidomastoid echo were defined as equal echo, those consistent with extravascular echo were defined as high echo, and those with high echo with sound shadow were defined as strong echo, that is, calcification. Plaques were defined as heterogenous when more than 20% of the echoes within the plaque were inconsistent. Ultrasonic diagnosis of carotid artery stenosis criteria: mild 40–49%, moderate 50–69%, severe 70–99%. Mild stenosis did not affect hemodynamics; moderate and severe stenosis affected carotid blood flow.
OCT examination methods
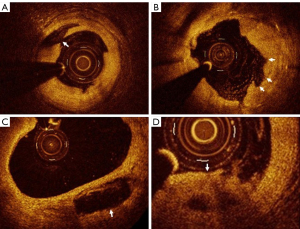
Frequency domain OCT was performed using a C7XR imaging system (St. Jude Medical Inc., St. Paul, MN, USA). OCT catheters were routinely delivered to the coronary artery before and after stent placement. All images were corrected, and a cross-sectional image was taken every 1.0 mm for analysis. The OCT diagnosis of the vulnerable plaques in the coronary artery was determined based on the histological definition of previously vulnerable plaques and the criteria for establishing vulnerable plaques (11). The criteria adopted in this study were as follows: active inflammation, a thin fibrous cap of <65 µm, a large fat nucleus (more than 40%, lipid area ≥ two quadrants), endothelial shedding with surface platelet aggregation, plaque fissure or lesion, and severe stenosis.
Statistical methods
The numerical data of a normal distribution were represented as mean ± standard deviation, and the Student’s t-test was used for inter group comparison. Classified data were expressed in frequency counts and percentages, and Chi-square test was used for intergroup comparison. The numerical data of non-normal distribution were represented as median and interquartile intervals, and Mann-Whitney U test was used for intergroup comparison.
Univariate and binary logistic regression analysis were conducted on the risk factors of vulnerable coronary artery plaques. Based on the results of univariate analysis with P values <0.05 and clinical significance, variables were introduced into multivariate analysis. The statistically significant difference was set as a bilateral P value <0.05. Receiver operating characteristic (ROC) curve analysis was used to identify the predictive ability of carotid plaque characteristics. Statistical analysis was performed using the software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc (version 15.2.2; MedCalc Software, Ostend, Belgium).
Results
Comparison of clinical data between the two groups of patients
Figure S1 displays the flow diagram of participants in the screening and analysis stages of this case-control study.
The results of the univariate analysis revealed that there were no significant differences in general data such as age, gender, BMI (kg/m2), smoking history, hypertension history, diabetes history, coronary artery stenosis <70%, and hyperlipidemia history between the two groups.
The differences in high-sensitivity CRP levels, coronary artery stenosis ≥70%, and carotid plaque characteristics (irregular plaque, heterogeneous plaque, hypoechoic plaque, plaque calcification, and a plaque thickness of >3 mm) between the two groups were statistically significant (see Table 1).
Table 1
| Items | Vulnerable plaque (n=35) | Stable plaque (n=35) | P value |
|---|---|---|---|
| Clinical data | |||
| Male/female | 26/9 | 19/16 | 0.068 |
| Age (years) | 49.12±0.63 | 47.12±0.27 | 0.239 |
| BMI (kg/m2) | 24.9±2.0 | 24.3±3.1 | 0.503 |
| Smoking history (n) | 21 | 17 | 0.053 |
| Hypertension history (n) | 26 | 22 | 0.054 |
| Diabetes history (n) | 17 | 15 | 0.723 |
| Hyperlipidemia history (n) | 17 | 14 | 0.252 |
| CRP (mg/L) | 3.6±1.1 | 3.1±1.5 | 0.002 |
| Carotid ultrasound findings (n) | |||
| Intima media thickening | 21 | 19 | 0.067 |
| Irregular plaque | 22 | 7 | 0.034 |
| Heterogeneous plaque | 18 | 17 | 0.002 |
| Hypoechoic plaque | 19 | 15 | 0.001 |
| Mixed echo plaques dominated with low echo | 7 | 18 | 0.079 |
| Calcified plaque | 11 | 9 | 0.034 |
| Plaque thickness >3 mm | 5 | 5 | 0.015 |
| Lumen stenosis ≥40% | 6 | 5 | 0.057 |
The numerical data of a normal distribution was represented as mean ± standard deviation, classified data was expressed in frequency counts. Smoking history: defined as a former smoker or a current smoker. Hypertension history: defined as a former hypertension patient or a current hypertension patient. Diabetes history: defined as a former diabetes patient or a current diabetes patient. BMI, body mass index; CRP, C-reactive protein.
The ultrasonic findings of different types of carotid plaques are displayed in Figure 1 and the OCT image findings of different types of coronary plaques are displayed in Figure 2.
Multivariate analysis and ROC curve analysis results
The variables with a statistical difference in the univariate analysis were included in the binary logistic regression equation, and the results revealed that an irregular fibrous cap of the carotid plaque and hypoechoic plaque were independent risk factors for predicting vulnerable plaques of the coronary artery (Table 2).
Table 2
| Variable | B | S.E. | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Irregular plaque | 1.573 | 0.794 | 3.918 | 4.819 | 1.106–22.867 | 0.048 |
| Hypoechoic plaque | 2.265 | 0.768 | 8.701 | 9.632 | 2.138–43.384 | 0.003 |
| Constant | −5.622 | 1.938 | 8.414 | 0.004 | – | 0.004 |
B, regression coefficient; S.E., standard error; OR, odds ratio; CI, confidence interval.
ROC curve analysis showed that the area under the curve (AUC) of the hypoechoic plaque for identifying coronary vulnerable plaques was 0.740 [95% confidence interval (CI): 0.591–0.889; P=0.006] with 68% sensitivity and 80% specificity. However, the AUCs of the irregular fibrous cap and carotid hypoechoic plaque combined with the irregular fibrous cap did not support their use for identification of coronary vulnerable plaque of SAP (P>0.05; Figure 3).

Discussion
The plaque fibrous cap is mainly composed of extracellular matrix (ECM) and smooth muscle cells. The synthesis and degradation of the ECM are regulated by multiple factors; therefore, the content in the fibrous cap reflects a dynamic process throughout the whole body, and this arterial change occurs in both the carotid and coronary plaques. When ECM synthesis decreases and degradation increases, the fibrous cap thins and weakens, making it easier to break (16).
The instability of a plaque is partly determined by local factors, and systemic factors such as infection, autoimmunity, or genes may also play important roles (17). If the stability of plaques is affected by systemic factors that exist only in a subset of patients, then plaques in some individuals should be more prone to rupture than those in others. A study revealed that patients with irregular plaques in the bilateral carotid arteries were more likely to have a history of myocardial infarction than those with smooth plaques [hazard ratio (HR) =1.82; 95% CI: 1.23–2.64; P<0.001] (17). Rossi et al.’s study revealed that coronary events (patients with acute myocardial infarction) predicted carotid vulnerable plaques [odds ratio (OR) =4.3; 95% CI: 2.0–9.2; P=0.0002] (18). This current study’s results revealed that both irregular carotid and hypoechoic plaques were independent risk factors for predicting vulnerable plaques of the coronary artery, which is consistent with the results of the above-mentioned studies. Therefore, although the carotid and coronary arteries are slightly different in the structural characteristics of their walls, anatomical positions, and hemodynamics, the results of this study demonstrated that a noninvasive carotid ultrasound was feasible in predicting vulnerable plaques of the coronary artery.
Although coronary angiography has been regarded as the gold standard for the diagnosis of coronary heart disease, it has limitations in evaluating the characteristics of the lumen walls and plaques and cannot observe vulnerable plaque characteristics associated with acute adverse cardiac events (18). Intravascular ultrasound (IVUS) and OCT can both identify vulnerable plaques, and their imaging mechanisms are similar (19). The main differences are the resolution and penetration depth; IVUS has deep penetration, and its primary advantage is assessing plaque load, whereas the penetration depth of OCT is limited (19). However, by imaging with near-infrared light, OCT can clearly display the characteristics of coronary plaque microstructure and quantify plaque composition with a spatial resolution of 10–20 mm (19). A study involving patients with acute myocardial infarction revealed that 73% of plaque ruptures could be observed by OCT, whereas only 40% could be observed by IVUS (20). Another study demonstrated that OCT was highly consistent with the histopathological control study and was recognized as “optical biopsy” (21). However, OCT is complex to operate, expensive, and has a narrow scope of use.
A carotid plaque is an indicator of atherosclerosis and is associated with the future risk of atherosclerotic cardiovascular disease (20). The location of the carotid artery is shallow, so ultrasound can clearly display its morphology, the echo of the carotid intima-media and any plaques, and whether the fibrous cap is smooth and flat (20). Therefore, as the most convenient and safe noninvasive screening method for atherosclerosis, carotid ultrasound has become a window through which atherosclerosis in the cardiovascular system can be visualized. It has also become commonly and widely used in clinical practice (20). Therefore, in this study, noninvasive carotid ultrasound was adopted for predicting vulnerable plaques of the coronary artery based on OCT images, which was of critical clinical significance. The echogenicity of atherosclerotic plaque mainly depends on aspects of its core such as lipids, necrotic elements of the lipid core, and the presence of microvascularization and mineralized or fibrous components on the core. Ultrasound images of lipid core and neovascularization in plaque showed low echo (22,23). The resolution of plaque hypoecho by human vision is significantly higher than the resolution of whether the plaque fiber cap is complete. In this study, plaque hypoecho was defined as lower than the sternocleidomastoid echo, so the results were more objective and accurate.
Patients with vulnerable plaque are suggested to undergo the following treatments: (I) control of risk factors: for example, hypertension patients need to undergo antihypertensive treatment, hyperglycemia patients need to control blood sugar, hyperlipidemia patients need to undergo lipid-lowering treatment, smoking patients need to quit smoking, overweight patients need to lose weight through weight control, lifestyle adjustments, and other methods. (II) Drug treatment: if the patient’s symptoms are relatively mild, they can be treated with oral statin drugs under the guidance of a doctor, such as atorvastatin calcium, rosuvastatin calcium, and pitavastatin calcium; they can also use coronary artery dilating drugs, such as nitroglycerin and nicodil, and aspirin enteric-coated tablets, among other drugs. (III) Surgical treatment: if the patient’s symptoms are relatively severe and the condition cannot be controlled by drugs, surgical treatment is required, such as PCI and CABG.
This study had some limitations. First, the sample selected was limited to SAP, and the sample size was too small to represent all patients with carotid atherosclerosis and coronary heart disease. In addition, the OCT examination was limited only to partial segments of the pathogenic vessels without a comprehensive examination of the coronary artery. Due to the significant gender difference in the occurrence of vulnerable plaques (24,25), the composition of males and females in the stable and vulnerable plaques groups was significantly different. Therefore, our results must be verified in a larger patient cohort.
Conclusions
Low-echo plaques detected by non-invasive carotid ultrasound can be used to predict coronary vulnerable plaques, but this needs to be confirmed by large-sample multi-center studies. Noninvasive carotid ultrasound is feasible and clinically valuable for predicting vulnerable and asymptomatic coronary plaques defined by OCT. With this method, adverse events can be diagnosed and treated in advance.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study. Thanks to Professor Yihua He, Director Jie Chen, and Director Qing Zhao from the Ultrasound Department of Anzhen Hospital for their guidance on this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-621/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-621/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Anzhen Hospital (No. 2022147X), informed consent provided by all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amato M, Veglia F, de Faire U, Giral P, Rauramaa R, Smit AJ, Kurl S, Ravani A, Frigerio B, Sansaro D, Bonomi A, Tedesco CC, Castelnuovo S, Mannarino E, Humphries SE, Hamsten A, Tremoli E, Baldassarre D. Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis 2017;263:412-9. [Crossref] [PubMed]
- Spence JD. Carotid plaque measurement is superior to IMT Invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis 2012;220:34-5. [Crossref] [PubMed]
- Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 2012;220:128-33. [Crossref] [PubMed]
- Nonin S, Iwata S, Sugioka K, Fujita S, Norioka N, Ito A, Nakagawa M, Yoshiyama M. Plaque surface irregularity and calcification length within carotid plaque predict secondary events in patients with coronary artery disease. Atherosclerosis 2017;256:29-34. [Crossref] [PubMed]
- Ishizu T, Ishimitsu T, Kamiya H, Seo Y, Moriyama N, Obara K, Watanabe S, Yamaguchi I. The correlation of irregularities in carotid arterial intima-media thickness with coronary artery disease. Heart Vessels 2002;17:1-6. [Crossref] [PubMed]
- Gaman SA, Balakhonova TV, Sinitsyn VE, At'kov OIu, Ternovoĭ SK. Structural and functional changes of coronary and carotid arteries in patients with ischemic heart disease. Ter Arkh 2005;77:15-21.
- Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis 2013;227:193-200. [Crossref] [PubMed]
- Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol 1997;146:483-94. [Crossref] [PubMed]
- Wattanakit K, Folsom AR, Chambless LE, Nieto FJ. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2005;149:606-12. [Crossref] [PubMed]
- Zamani M, Skagen K, Scott H, Lindberg B, Russell D, Skjelland M. Carotid Plaque Neovascularization Detected With Superb Microvascular Imaging Ultrasound Without Using Contrast Media. Stroke 2019;50:3121-7. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation 2003;108:1772-8. [Crossref] [PubMed]
- Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA 1998;279:119-24. [Crossref] [PubMed]
- Jones NR, McCormack T, Constanti M, McManus RJ. Diagnosis and management of hypertension in adults: NICE guideline update 2019. Br J Gen Pract 2020;70:90-1. [Crossref] [PubMed]
- 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol 2018;15:1-29. [Crossref] [PubMed]
- Lian F, Ni Q, Shen Y, Yang S, Piao C, Wang J, Wei J, Duan J, Fang Z, Lu H, Yang G, Zhao L, Song J, Li Q, Zheng Y, Lyu Y, Tong X. International traditional Chinese medicine guideline for diagnostic and treatment principles of diabetes. Ann Palliat Med 2020;9:2237-50. [Crossref] [PubMed]
- Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ 2013;22:399-411. [Crossref] [PubMed]
- Rothwell PM, Villagra R, Gibson R, Donders RC, Warlow CP. Evidence of a chronic systemic cause of instability of atherosclerotic plaques. Lancet 2000;355:19-24. [Crossref] [PubMed]
- Rossi A, Franceschini L, Fusaro M, Cicoira M, Eleas AA, Golia G, Bonapace S, Santini F, Sangiorgi G, Zardini P, Vassanelli C. Carotid atherosclerotic plaque instability in patients with acute myocardial infarction. Int J Cardiol 2006;111:263-6. [Crossref] [PubMed]
- Nicholls SJ, Nissen SE, Prati F, Windecker S, Kataoka Y, Puri R, Hucko T, Kassahun H, Liao J, Somaratne R, Butters J, Di Giovanni G, Jones S, Psaltis PJ. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc Diagn Ther 2021;11:120-9. [Crossref] [PubMed]
- Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol 2007;50:933-9. [Crossref] [PubMed]
- Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol 2007;50:933-9. [Crossref] [PubMed]
- Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol 2006;97:1172-5. [Crossref] [PubMed]
- Langsfeld M, Gray-Weale AC, Lusby RJ. The role of plaque morphology and diameter reduction in the development of new symptoms in asymptomatic carotid arteries. J Vasc Surg 1989;9:548-57. [Crossref] [PubMed]
- Gasbarrino K, Di Iorio D, Daskalopoulou SS. Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur Heart J 2022;43:460-73. [Crossref] [PubMed]
- Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol 2006;47:C7-12. [Crossref] [PubMed]


