
Development and validation of a clinical factors and body fat distribution-based nomogram to predict refractoriness of transarterial chemoembolization in hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor worldwide and has the second highest mortality rate among malignant tumors (1). According to the Barcelona Clinic Liver Cancer (BCLC) staging system, transarterial chemoembolization (TACE) is considered the standard treatment for patients with intermediate stage HCC (2). HCC usually requires multiple repetitions of TACE therapy to control tumor progression (3). The concept of TACE-refractory/failed was first introduced by the Japanese Society of Hepatology (JSH) and revised in 2014 and 2021 (4,5). The revised definition of TACE refractoriness by the JSH has been accepted in several Asian clinical treatment guidelines. The concept of TACE failure/refractory has been widely introduced in clinical trials of HCC (6,7).
Recently, several studies have begun to focus on the effect of body composition on the prognosis of patients with HCC (8-11). Computed tomography (CT) allows quantitative measurement and adequate differentiation of body composition, such as skeletal muscle (SM), subcutaneous fat, visceral fat, etc. and is considered an important technique for assessing body composition (12). The body composition parameters that have been studied more frequently are SM and adipose tissue, and changes in these body composition parameters have been associated with poor prognosis in patients with tumors, including HCC (13-15). To our knowledge, studies on the possible correlation between SM and adipose tissue and TACE refractoriness in patients with HCC are still lacking.
Repeated TACE therapy is associated with increased angiogenesis and embolization-related liver injury, and may even counteract the benefits achieved by TACE therapy in tumors and adversely affect overall survival (16,17). It may be undesirable for TACE-refractory patients to pursue repeated TACE to control tumor progression in clinical practice. Therefore, it is important to carefully assess the indications for TACE and appropriately identify TACE failure/refractoriness to prevent deterioration of liver function due to ineffective TACE.
In this study, we focused on the effect of body composition on refractoriness to TACE treatment for HCC. Our goal was to develop and validate a noninvasive and easily applied predictive model that can accurately predict the risk of TACE refractoriness preoperatively. We present this article in accordance with the TRIPOD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-963/rc).
Methods
Patients
This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Hunan Cancer Hospital. Informed consent was waived because the data of patients were collected retrospectively. All patients’ data were anonymized before analysis. This study retrospectively screened 745 patients diagnosed with HCC and treated with TACE between June 2013 and October 2021 in Hunan Cancer Hospital. Based on the inclusion and exclusion criteria, a total of 128 patients were finally included in the study, and the clinical data of all patients were collected. Inclusion criteria were as follows: (I) HCC diagnosed by pathological biopsy, cytology or imaging according to the diagnostic criteria for HCC established by the American College of Hepatology; (II) 18–75 years of age; (III) receiving TACE treatments; (IV) patients with BCLC stage A or B; (V) and enhanced CT examination within two weeks before the first TACE procedure.
Exclusion criteria were as follows: (I) incomplete clinical and imaging data; (II) initial treatment was not TACE; (III) time interval between the first and second TACE was more than 3 months; (IV) combination of other antitumor treatments; (V) extrahepatic metastasis or combination of malignant tumors from other sites; (VI) missed visits. The study flow is shown in Figure 1.

Treatment
TACE was performed within two weeks of the patient’s diagnosis of HCC, and all patients were treated with conventional TACE. All procedures were performed by interventionalists with over 10 years of experience. Using the Seldinger technique, a 5 F arterial catheter was inserted into the femoral artery after local anesthesia. The catheter was then advanced into the hepatic artery, and digital subtraction angiography was performed. All patients underwent abdominal trunk and superior mesenteric artery angiography to assess hepatic vascular circulation prior to treatment. Tumor trophoblastic vessels were hyperselected using 2.7 F or 2.2 F microcatheters, if necessary, depending on liver involvement and vascular anatomy. TACE was performed using an oil iodide emulsion containing an epirubicin mix. TACE was performed using 20 mL of iodinated oil emulsion containing a mixture with epirubicin. Using the water-in-oil technique, the oil-epirubicin emulsion is prepared by mixing iodinated oil with a distilled water solution containing the dissolved epirubicin drug mixture in a 3:1 ratio. The dose of epirubicin in conventional TACE is 50–75 mg/m2. in conventional TACE, after injection of epirubicin oil emulsion, gelatin sponge slurry is injected to embolize the proximal tumor vessels. The technical endpoint of TACE was defined as a decrease in tumor artery inflow and tumor vessel dissection and loss of tumor staining. When there was an inadequate response after the first TACE procedure, the embolic agent, chemotherapeutic agent or tumor donor artery was reselected in the second TACE procedure, and the protocol was adjusted according to liver function and peripheral leukocyte and platelet levels.
Definition and follow-up of TACE refractoriness
The JSH revised definition of TACE refractoriness in 2021 states the following (5): (I) 2 or more consecutive hepatic progressions with poor target tumor response (viable lesion >50%) or new tumor lesions at CT/magnetic resonance imaging (MRI) response assessment 1 to 3 months after elective TACE even with chemotherapy drug change and/or reconfirmation of tumor arteries; (II) extrahepatic metastases or vascular invasion; (III) a decrease in tumor marker level is not observed immediately after TACE, or only a minimal and transient decrease is observed, but a trend of increasing tumor marker levels immediately follows the procedure.
Enhanced CT or MRI images and clinical data were obtained for all patients before and after the first and second TACE treatments. Two abdominal radiologists with extensive experience who were not involved in body composition analysis evaluated all available follow-up CT or MRI images 1–3 months after TACE to determine the response to TACE treatment for HCC.
CT scan analysis of body composition
Enhanced CT scans of the abdomen were performed within two weeks before/after the first TACE treatment, pre-treatment images were used for analysis, and the abdominal CT image parameters were as follows: 5-mm layer thickness, 120 kVp, 250 mA, and 40 cm field of view. The CT image data of all patients were stored in the picture archiving and communication system and output in DICOM format.
All patients were randomly divided into a training cohort and a validation cohort. The areas of SM, visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and intermuscular adipose tissue (IMAT) at the level of L3 on CT images in the venous phase were measured separately in this study using ImageJ software (https://imagej.nih.gov/ij/). The area of SM at the level of third lumbar vertebra included the psoas major, rectus abdominis internal and external oblique muscles as well as the transverse abdominis, lumbar square and erector spinae muscles. Heinz unit (HU) thresholds were used to differentiate body component tissues: −29 to +150 HU for SM, −190 to −30 HU for subcutaneous and intermuscular fat, and −150 to −50 HU for visceral fat (Figure 2). The cross-sectional area of each variable was then normalized by dividing by the square of the patient’s height (m2) to obtain an index (cm2/m2). The skeletal muscle index (SMI) was calculated as the area of SM at the third lumbar vertebra level divided by the square of height (cm2/m2). The visceral adipose tissue index (VATI), subcutaneous adipose tissue index (SATI), and intermuscular adipose tissue index (IMATI) were defined as the adipose tissue area divided by the square of the height (cm2/m2). The total adipose tissue index (TATI) was defined as the sum of VATI and SATI, and visceral-to-subcutaneous adipose tissue area ratio (VSR) was defined as the ratio of VAT to SAT area.
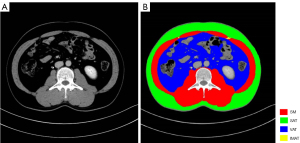
Two trained radiologists measured and assessed SM, SAT, VAT, and IMAT together and discussed until agreement was reached on each assessment.
Statistical analysis
Continuous variables in patient clinical data were expressed as the mean and standard deviation or median and interquartile range, and categorical variables were expressed as percentages. Continuous variables between the two groups were tested using the t-test or the Mann-Whitney U test. Categorical variables were tested using the chi-square test. Univariate and multivariate analyses were performed to confirm clinical factors for TACE refractoriness. Clinical factors with P<0.05 in the univariate logistic regression analysis were included in the multivariate analysis, and independent predictors of TACE refractoriness were identified and modeled in the multivariate logistic regression analysis. Multiple model prediction accuracy in the training cohort and validation cohort was quantified by the area under the curve (AUC). Nomograms were used to visualize the prediction models and calculate the risk of occurrence for each patient, and calibration curves and decision curves were used to evaluate model performance. Patients were grouped into high- and low-risk groups for subgroup analysis based on the receiver operator characteristic (ROC) curve best cutoff values. Statistical analyses were performed using R software (version 3.6.3, http://www.r-project.org). All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Baseline characteristics of the patient’s clinical and body composition
The 128 patients with HCC treated with TACE were randomly divided into training and validation cohorts, and all TACE procedures were technically successful. Table 1 summarizes the training cohort (n=89) and the validation cohort (n=39) as well as patient clinical data and body composition characteristics. The mean age of all patients was 56 years, and the majority of patients were male (88.3%). There was no significant difference between the training and validation cohorts for the remaining variables except for the significant difference between the training and validation cohorts for satellite nodules on CT images (P=0.031). The total number of patients included 56 (43.8%) TACE-refractory patients and 72 (56.2%) non-TACE-refractory patients. The training and validation cohorts included 36 (40.4%) and 20 (51.3%) TACE-refractory patients, respectively, and there was no significant difference in the prevalence of TACE between the two groups (P=0.35).
Table 1
| Characteristic | All patients (N=128) | Training cohort (N=89) | Validation cohort (N=39) | P value |
|---|---|---|---|---|
| Age (years) | 56±11 | 56±11 | 55±11 | 0.58 |
| Gender | 0.97 | |||
| Female | 15 (11.7) | 11 (12.4) | 4 (10.3) | |
| Male | 113 (88.3) | 78 (87.6) | 35 (89.7) | |
| ALT (U/L) | 38.9 (27.7–60.0) | 37.3 (27.0–57.6) | 47.2 (34.0–62.7) | 0.06 |
| AST (U/L) | 49.4 (36.4–70.9) | 46.0 (36.5–67.5) | 56.2 (35.5–84.5) | 0.14 |
| Albumin (g/L) | 38.1±4.8 | 38.5±4.6 | 37.2±5.3 | 0.17 |
| TB (μmol/L) | 15.5 (12.5–22.0) | 15.8 (12.6–21.9) | 15.3 (12.3–20.9) | 0.66 |
| PT (s) | 13.2 (12.4–14.1) | 13.2 (12.5–14.0) | 13.3 (12.1–14.4) | 0.84 |
| NLR | 2.8 (1.9–4.3) | 2.9 (1.9–4.5) | 2.8 (1.7–4.0) | 0.47 |
| Tumor size (cm) | 6.5 (5.8–9.9) | 7.3 (5.8–9.5) | 6.0 (5.1–8.6) | 0.25 |
| Child-Pugh grade | 0.73 | |||
| A | 115 (89.8) | 81 (91.0) | 34 (87.2) | |
| B | 13 (10.2) | 8 (9.0) | 5 (12.8) | |
| ECOG | 0.46 | |||
| 0 | 61 (47.7) | 40 (44.9) | 21 (53.8) | |
| 1 | 67 (52.3) | 49 (55.1) | 18 (46.2) | |
| HBV | 0.77 | |||
| Negative | 13 (10.2) | 10 (11.2) | 3 (7.7) | |
| Positive | 115 (89.8) | 79 (88.8) | 36 (92.3) | |
| AFP (ng/mL) | 0.64 | |||
| ≤100 | 60 (46.9) | 40 (44.9) | 20 (51.3) | |
| >100 | 68 (53.1) | 49 (55.1) | 19 (48.7) | |
| Satellite | 0.035 | |||
| Negative | 98 (76.6) | 63 (70.8) | 35 (89.7) | |
| Positive | 30 (23.4) | 26 (29.2) | 4 (10.3) | |
| Tumor number | 0.10 | |||
| Single | 60 (46.9) | 37 (41.6) | 23 (59.0) | |
| Multiple | 68 (53.1) | 52 (58.4) | 16 (41.0) | |
| Cirrhosis | 0.38 | |||
| Negative | 65 (50.8) | 48 (53.9) | 17 (43.6) | |
| Positive | 63 (49.2) | 41 (46.1) | 22 (56.4) | |
| BCLC | 0.19 | |||
| A | 9 (7.0) | 4 (4.5) | 5 (12.8) | |
| B | 119 (93.0) | 85 (95.5) | 34 (87.2) | |
| VATI (cm2/m2) | 29.7 (17.1–48.1) | 29.0 (16.4–48.1) | 31.6 (20.3–48.4) | 0.73 |
| SATI (cm2/m2) | 31.9 (19.1–47.4) | 30.0 (18.2–44.2) | 35.0 (21.2–49.9) | 0.43 |
| VSR (cm2/m2) | 0.92 (0.67–1.34) | 0.93 (0.66–1.35) | 0.91 (0.70–1.27) | 0.77 |
| TATI (cm2/m2) | 64.4 (37.7–92.2) | 62.2 (36.9–93.0) | 68.8 (44.4–91.7) | 0.55 |
| SMI (cm2/m2) | 47.6±7.4 | 47.7±7.4 | 47.4±7.5 | 0.82 |
| IMATI (cm2/m2) | 2.4 (1.5–4.0) | 2.3 (1.6–3.7) | 2.9 (1.4–4.3) | 0.72 |
| TACE refractoriness | 0.35 | |||
| No | 72 (56.2) | 53 (59.6) | 19 (48.7) | |
| Yes | 56 (43.8) | 36 (40.4) | 20 (51.3) |
Continuous variables are expressed as means (± standard deviations) or medians (interquartile ranges), and categorical variables are expressed as numbers (percentages). ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; PT, prothrombin time; NLR, neutrophil-to-lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; VATI, visceral adipose tissue index; SATI, subcutaneous adipose tissue index; VSR, visceral-to-subcutaneous adipose tissue area ratio; TATI, total adipose tissue index; SMI, skeletal muscle index; IMATI, intermuscular adipose tissue index; TACE, transarterial chemoembolization.
Independent risk factors for TACE refractoriness
Figure 3 shows a case of a typical TACE-refractory patient. The results of univariate and multivariate logistic analyses of clinical data and body composition of the training cohort are shown in Table 2. The results of the univariate analysis showed the following factors with P<0.05: tumor size (P<0.001), alpha-fetoprotein (AFP) >100 (ng/mL) (P=0.002), cirrhosis (P=0.049), VATI (P=0.013), and VSR (P<0.001). Further multivariate logistic analysis revealed that tumor size [P=0.001; odds ratio (OR): 1.55, 95% confidence interval (CI): 1.2–2.01], AFP >100 ng/mL (P=0.041; OR: 3.31, 95% CI: 1.01–10.87), and VSR (P=0.043; OR: 4.04, 95% CI: 1.02–16.04) were independent predictors of TACE refractoriness in HCC patients.

Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age (years) | 0.99 (0.95–1.03) | 0.61 | |||
| Gender | |||||
| Female | 1 | ||||
| Male | 1.22 (0.33–4.51) | 0.77 | |||
| Albumin | 1.02 (0.93–1.11) | 0.75 | |||
| ALT | 1.00 (0.98–1.01) | 0.54 | |||
| AST | 0.99 (0.98–1.01) | 0.33 | |||
| Tumor size (cm) | 1.68 (1.32–2.13) | <0.001 | 1.55 (1.2–2.01) | 0.001 | |
| AFP (ng/mL) | |||||
| ≤100 | 1 | ||||
| >100 | 4.23 (1.67–10.73) | 0.002 | 3.31 (1.01–10.87) | 0.041 | |
| Tumor number | |||||
| Single | 1 | ||||
| Multiple | 2.19 (0.9–5.33) | 0.08 | |||
| Cirrhosis | |||||
| Negative | 1 | ||||
| Positive | 0.41 (0.17–1.00) | 0.049 | 0.72 (0.22–2.28) | 0.57 | |
| BCLC | |||||
| A | 1 | ||||
| B | 0.67 (0.09–4.96) | 0.69 | |||
| VATI | 1.03 (1.01–1.05) | 0.013 | 1.01 (0.98–1.04) | 0.36 | |
| SATI | 1 (0.98–1.03) | 0.88 | |||
| VSR | 9.87 (3.13–31.1) | <0.001 | 4.04 (1.02–16.04) | 0.043 | |
| TATI | 1.01 (1–1.02) | 0.13 | |||
| SMI | 1.01 (0.95–1.07) | 0.84 | |||
| IMATI | 0.98 (0.76–1.26) | 0.86 | |||
“1” means reference value. CI, confidence interval; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; VATI, visceral adipose tissue index; SATI, subcutaneous adipose tissue index; VSR, visceral-to-subcutaneous adipose tissue area ratio; TATI, total adipose tissue index; SMI, skeletal muscle index; IMATI, intermuscular adipose tissue index.
Clinical and body composition model construction
AFP level, tumor size, and VSR were used as independent risk factors to construct prediction models and plot ROC curves separately. The AUC values for AFP in the training cohort and validation cohort were 0.667 (95% CI: 0.569–0.766) and 0.770 (95% CI: 0.634–0.905), respectively. The AUC values for tumor size in the training cohort and validation cohort were 0.827 (95% CI: 0.743–0.911) and 0.778 (95% CI: 0.620–0.935), respectively. The AUC values for VSR in the training cohort and validation cohort were 0.793 (95% CI: 0.699–0.887) and 0.733 (95% CI: 0.566–0.900), respectively.
Construction and evaluation of a combined clinical-body composition model
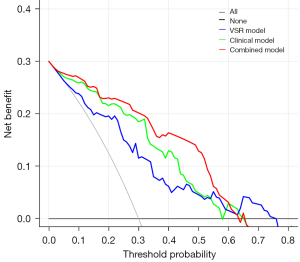
The AFP level, tumor size, and VSR were combined to construct a combined clinical-body composition prediction model and plot ROC curves (Figure 4A,4B), and the combined model performed best in predicting TACE refractoriness in both the training and validation cohorts. The combined model had an AUC value of 0.875 (95% CI: 0.802–0.949) with a sensitivity of 83.3% and specificity of 89.5% in the training cohort and an AUC value of 0.837 (95% CI: 0.705–0.969) with a sensitivity of 75.0% and specificity of 84.9% in the validation cohort. The performance of the different models to predict TACE refractoriness in the training and validation cohorts is shown in Table 3. The calibration curve showed good agreement between the training and validation cohorts in predicting the occurrence of TACE refractoriness and the true situation (Figure 4C,4D). The nomogram visualized the prediction model (Figure 5), and the risk scores of the relevant variables can be calculated from the baseline situation of the patient before TACE treatment with the nomogram. Finally, the risk of refractory outcome after TACE treatment is determined from the total score. Decision curve analysis (DCA) showed that the net clinical benefit of the combined clinical-body composition model was higher than that of either the clinical model (AFP level and tumor size) or the body composition VSR model alone over a wide range of threshold probability intervals (Figure 6).
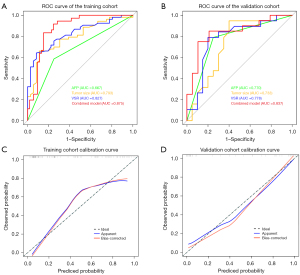
Table 3
| Models | Cohort | Sensitivity | Specificity | Accuracy | AUC (95% CI) |
|---|---|---|---|---|---|
| AFP | Training cohort | 0.75 | 0.789 | 0.652 | 0.667 (0.569–0.766) |
| Validation cohort | 0.75 | 0.585 | 0.763 | 0.770 (0.634–0.905) | |
| Tumor size | Training cohort | 0.917 | 0.789 | 0.795 | 0.827 (0.743–0.911) |
| Validation cohort | 0.8 | 0.642 | 0.753 | 0.778 (0.620–0.935) | |
| VSR | Training cohort | 0.889 | 0.860 | 0.769 | 0.793 (0.699–0.887) |
| Validation cohort | 0.6 | 0.717 | 0.724 | 0.733 (0.566–0.900) | |
| Combined model | Training cohort | 0.833 | 0.895 | 0.843 | 0.875 (0.802–0.949) |
| Validation cohort | 0.75 | 0.849 | 0.821 | 0.837 (0.705–0.969) |
AUC, area under the curve; CI, confidence interval; AFP, alpha-fetoprotein; VSR, visceral-to-subcutaneous adipose tissue area ratio.

Subgroup analysis
The ROC based on the combined model of the training cohort determined the optimal cutoff value of 65.7 for the Youden index. The total score for each patient was determined and stratified to represent high- and low-risk groups based on the cutoff value. Patients with a total score <65.7 in both the training cohort and the validation cohort were considered the low-risk subgroup, and those with a score ≥65.7 were considered the high-risk subgroup. The difference in prevalence of TACE-refractoriness between the low-risk and high-risk subgroups was statistically significant in the training cohort (18.9% vs. 88.9%; P<0.001) and the validation cohort (30% vs. 94.7%; P<0.001) (Table 4). We found that the prevalence of TACE refractoriness was higher in all high-risk groups than in the low-risk group, suggesting that the combined prediction model can help identify and guide the clinical management of high-risk TACE-refractory patients.
Table 4
| Groups | Without TACE refractoriness, n (%) | With TACE refractoriness, n (%) | P value |
|---|---|---|---|
| Training cohort | |||
| High-risk group | 10 (18.9) | 32 (88.9) | <0.001 |
| Low-risk group | 43 (81.1) | 4 (11.1) | |
| Validation cohort | |||
| High-risk group | 6 (30.0) | 18 (94.7) | <0.001 |
| Low-risk group | 14 (70.0) | 1 (5.3) |
TACE, transarterial chemoembolization.
Discussion
Our study constructed a noninvasive, convenient, and easy-to-use model to preoperatively predict the risk of TACE refractoriness in HCC patients after TACE. To the best of our knowledge, our study is the first to propose that TACE refractoriness in HCC patients is associated with body composition VSR. The prediction model performed better in both the training cohort and the validation cohort. Based on these results, our model can help to appropriately select patients for TACE treatment, and make more rational and scientific decisions about TACE treatment; making the decision to switch to molecular targeted therapy or combination therapy earlier for patients at risk of TACE refractoriness is facilitated.
TACE is the standard of care for intermediate-stage HCC. However, the effectiveness of this treatment may be limited by the possibility of patients developing TACE refractoriness (18). According to JSH recommendations, ineffective TACE should not be repeated in patients who develop TACE refractoriness, and it is recommended that these patients be treated with systemic therapy (4). Therefore, patients at high risk of TACE refractoriness can be rapidly identified for timely conversion to combination therapy or molecular targeted therapy. Several scoring systems have been developed to predict TACE refractoriness (19). One study established a scoring system called the Assessment for Retreatment with TACE (ART) score. The ART scores included the increase of aspartate aminotransferase by >25%, an increase of Child-Pugh score of 1 or ≥2 points from baseline, and the absence of radiologic tumor response. Patients with an ART score ≥ 2.5 before the second TACE have a poor prognosis and may not benefit from further TACE therapy. However, given that radiologic response is a parameter of the ART score, prospective studies are needed to validate the ART score and incorporate the Modified Response Evaluation Criteria In Solid Tumors into the study design (20). Another study also constructed an included BCLC and AFP (>200 ng/mL) at baseline, increase in Child-Pugh score ≥2 from baseline, and absence of radiological response (ABCR) score. ABCR scores were significantly associated with median overall survival. An ABCR score >4 before a second TACE identifies patients with a poor prognosis who may not benefit from further TACE treatment. However, the ABCR score has limitations and it should be tested in different populations and validated in prospective trials (21). However, one study noted that neither score is sufficient to support definitive clinical decision making and that further efforts are needed to assess TACE refractoriness (22). Therefore, preoperative quantitative prediction of the risk of TACE refractoriness can provide critical information to guide decision making regarding TACE treatment in HCC patients.
In recent years, high VAT seemingly correlating with a risk factor for HCC in male patients with cirrhosis and for recurrence after liver transplantation (23). Sarcopenia has been one of the most evaluated body composition-related conditions, and its presence has been shown to be associated with poor prognosis, recurrence and overall complications in patients with HCC (8,24,25). However, the influence of body composition on the disease process still needs to be further explored. To address this question, we combined clinical factors and measures of multiple body compositions in HCC patients to assess the risk of TACE refractoriness occurring. Regarding clinical factors, AFP and tumor size were associated with TACE refractoriness. Previous studies have shown that neutrophil-to-lymphocyte ratio (NLR) is a widely validated indicator of prognostic information for patients with HCC (26). Although NLR did not lead to this conclusion in our study, we also need to recognize the important role of NLR in the treatment as well as prognosis of HCC. We need to further explore the importance of NLR in future studies.
For body composition, the results showed that VSR was an independent risk factor associated with TACE refractoriness. The prediction model based on three independent risk factors including VSR further improved the predictive performance of clinical factors, and the combined model of the three had the optimal predictive performance, with AUCs of 0.875 (95% CI: 0.802–0.949) and 0.837 (95% CI: 0.705–0.969) in the training and validation cohorts, respectively. The calibration curves indicated that the combined model was a robust prognostic model. The DCA indicated that the combined model had high clinical application. According to the cutoff values of the model risk scores, patients in the high-risk group were more likely to develop TACE refractoriness than those in the low-risk group, suggesting that the model contributes to the risk classification of patients with TACE refractoriness. Based on these results, both clinical and body composition factors are indispensable for predicting TACE refractoriness.
For patients who are refractory to TACE, we think that the subsequent treatment should be different for patients with different conditions. If the internal target lesion is still in progression disease after two TACE treatments, it may be necessary to discontinue TACE and switch to other treatments, including systemic therapies such as targeted therapies, immune checkpoint inhibitor therapies, and combinations of other local therapies such as hepatic arterial infusion chemotherapy, transarterial radioembolization (Y90), ablation, and 125I particle implantation. If vascular invasion or extrahepatic spread occurs after TACE, systemic therapy, such as targeted therapy combined with immunotherapy, is required. If vascular invasion or extrahepatic spread occurs after TACE, continuation of TACE is important to control the intrahepatic lesions, and a combination of other local therapies including TACE, such as ablation, transarterial radioembolization (Y90), and particle therapy, may be used.
In our study, there was no significant effect of SMI on the response to TACE therapy in HCC, and studies in larger cohorts may be needed to validate the relationship between the two. Our results showed that a higher VSR was associated with TACE refractoriness in patients with HCC, whereas VATI and SATI were not. This finding suggests that the distribution of adipose tissue, rather than the absolute value, is the main determinant of TACE treatment response in patients with HCC. Adipose tissue controls the function of other organs by secreting adipokines. There are significant differences in cytokine production between VAT and SAT (27). The accumulation of visceral fat increases the secretion of proinflammatory adipokines such as tumor necrosis factor-α and interleukin 6 by visceral adipocytes and decreases the level of the anti-inflammatory adipokine lipocalin (28), whereas the anti-inflammatory cytokine lipocalin is secreted by subcutaneous adipocytes (29). These results suggest that the balance between visceral and SAT is more important than VATI itself. Since free fatty acids and adipokines released from visceral fat flow directly to the liver through the portal vein, the liver is substantially affected by such changes (30). In contrast, subcutaneous fat effectively stores excess lipids and fats to prevent them from entering other organs. However, the present study cannot yet address the question of causality, and further basic and clinical studies are needed.
Our study still has some limitations: (I) this is a retrospective study with a small sample size and, therefore, may be subject to selection and statistical bias. (II) Due to the small sample size of the study and the relatively low clinical incidence of TACE refractoriness, a more detailed analysis was not possible, and a subsequent multicenter, large sample size prospective study is needed for further validation before more convincing conclusions can be drawn. (III) This study was observational; therefore, we cannot infer a causal relationship between body composition and TACE refractoriness. (IV) Our patients and results may not be applicable to HCC patients in non-hepatitis B populations, while more validation is needed. (V) Our choice of AFP ≥100 ng/mL may have some limitations, so we need to further explore the effect of different AFP levels on HCC TACE refractoriness in future studies.
Conclusions
In summary, our study provides a reliable, and noninvasive method to predict TACE refractoriness in patients with HCC before TACE treatment. This will likely help clinicians select timely modalities such as combination therapy or molecular targeted therapy and, thereby, improve the prognosis of patients with HCC after TACE therapy.
Acknowledgments
Funding: This work received support from the following funding sources
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-963/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-963/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Hunan Cancer Hospital. Informed consent was waived because the data of patients were collected retrospectively.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raoul JL, Edeline J. Systemic treatment of hepatocellular carcinoma: standard of care in China and elsewhere. Lancet Oncol 2020;21:479-81. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol 2017;67:173-83. [Crossref] [PubMed]
- Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami TLiver Cancer Study Group of Japan. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014;87:22-31. [Crossref] [PubMed]
- Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021;10:181-223. [Crossref] [PubMed]
- Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y, Sakurai T, Nishida N, Kudo M. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2015;4:253-62. [Crossref] [PubMed]
- Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. [Crossref] [PubMed]
- Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131-40. [Crossref] [PubMed]
- Labeur TA, van Vugt JLA, Ten Cate DWG, Takkenberg RB, IJzermans JNM, Groot Koerkamp B, de Man RA, van Delden OM, Eskens FALM, Klümpen HJ. Body Composition Is an Independent Predictor of Outcome in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Liver Cancer 2019;8:255-70. [Crossref] [PubMed]
- Grąt K, Pacho R, Grąt M, Krawczyk M, Zieniewicz K, Rowiński O. Impact of Body Composition on the Risk of Hepatocellular Carcinoma Recurrence After Liver Transplantation. J Clin Med 2019;8:1672. [Crossref] [PubMed]
- Parikh ND, Zhang P, Singal AG, Derstine BA, Krishnamurthy V, Barman P, Waljee AK, Su GL. Body Composition Predicts Survival in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Cancer Res Treat 2018;50:530-7. [Crossref] [PubMed]
- Pickhardt PJ, Summers RM, Garrett JW. Automated CT-Based Body Composition Analysis: A Golden Opportunity. Korean J Radiol 2021;22:1934-7. [Crossref] [PubMed]
- Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJ, Goh V. Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015;6:489-97. [Crossref] [PubMed]
- Imai K, Takai K, Miwa T, Maeda T, Hanai T, Shiraki M, Suetsugu A, Shimizu M. Increased Visceral Adipose Tissue and Hyperinsulinemia Raise the Risk for Recurrence of Non-B Non-C Hepatocellular Carcinoma after Curative Treatment. Cancers (Basel) 2021;13:1542. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yao S, Yagi S, Kamo N, Seo S, Taura K, Okajima H, Uemoto S. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2019;8:92-109. [Crossref] [PubMed]
- Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, Takita M, Hagiwara S, Minami Y, Ida H, Takenaka M, Sakurai T, Watanabe T, Morita M, Ogawa C, Wada Y, Ikeda M, Ishii H, Izumi N, Nishida N. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel) 2019;11:1084. [Crossref] [PubMed]
- Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37:212-20. [Crossref] [PubMed]
- Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020;9:245-60. [Crossref] [PubMed]
- Chen L, Yu CX, Zhong BY, Zhu HD, Jin ZC, Zhu GY, Zhang Q, Ni CF, Teng GJ. Development of TACE Refractoriness Scores in Hepatocellular Carcinoma. Front Mol Biosci 2021;8:615133. [Crossref] [PubMed]
- Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Müller C, Heinzl H, Trauner M, Peck-Radosavljevic M. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2013;57:2261-73. [Crossref] [PubMed]
- Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, Monnet O, Beaurain P, Bazin C, Pol B, Folgoc GL, Castellani P, Bronowicki JP, Bourlière M. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol 2015;62:855-62. [Crossref] [PubMed]
- Kloeckner R, Pitton MB, Dueber C, Schmidtmann I, Galle PR, Koch S, Wörns MA, Weinmann A. Validation of Clinical Scoring Systems ART and ABCR after Transarterial Chemoembolization of Hepatocellular Carcinoma. J Vasc Interv Radiol 2017;28:94-102. [Crossref] [PubMed]
- Montano-Loza AJ, Mazurak VC, Ebadi M, Meza-Junco J, Sawyer MB, Baracos VE, Kneteman N. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology 2018;67:914-23. [Crossref] [PubMed]
- Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, Kamo N, Yagi S, Taura K, Okajima H, Uemoto S. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2019;269:924-31. [Crossref] [PubMed]
- Meister FA, Lurje G, Verhoeven S, Wiltberger G, Heij L, Liu WJ, Jiang D, Bruners P, Lang SA, Ulmer TF, Neumann UP, Bednarsch J, Czigany Z. The Role of Sarcopenia and Myosteatosis in Short- and Long-Term Outcomes Following Curative-Intent Surgery for Hepatocellular Carcinoma in a European Cohort. Cancers (Basel) 2022;14:720. [Crossref] [PubMed]
- Shelat VG. Role of inflammatory indices in management of hepatocellular carcinoma-neutrophil to lymphocyte ratio. Ann Transl Med 2020;8:912. [Crossref] [PubMed]
- Marra F, Bertolani C. Adipokines in liver diseases. Hepatology 2009;50:957-69. [Crossref] [PubMed]
- Arano T, Nakagawa H, Tateishi R, Ikeda H, Uchino K, Enooku K, Goto E, Masuzaki R, Asaoka Y, Kondo Y, Goto T, Shiina S, Omata M, Yoshida H, Koike K. Serum level of adiponectin and the risk of liver cancer development in chronic hepatitis C patients. Int J Cancer 2011;129:2226-35. [Crossref] [PubMed]
- Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G, Palombo D, Arsenescu R, Arsenescu V. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 2014;37:1337-53. [Crossref] [PubMed]
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582-8. [Crossref] [PubMed]


