
Pulmonary extranodal natural killer/T-cell lymphoma: clinical presentation, CT characteristics, and patient outcomes
Introduction
Extranodal natural killer/T-cell lymphoma (ENKTCL) is a subtype of non-Hodgkin’s lymphoma that originates from activated NK cells or cytotoxic T cells. ENKTCL is strongly associated with Epstein-Barr virus (EBV) infection and occurs more frequently in eastern Asia and Latin America compared to Europe (1,2). ENKTCL tends to invade various organs or tissues, with the nasal cavity being the most affected sites, followed by the skin and digestive system (3,4). Lung involvement in ENKTCL is extremely rare, making it one of the least studied types of lymphoma (5,6).
Both primary pulmonary ENKTCL and secondary pulmonary ENKTCL are characterized by its invasive nature and poor prognosis (7). Diagnosis can be challenging due to its nonspecific symptoms, which include fever, cough, and dyspnea. Chest computed tomography (CT) scans often show unspecific findings such as multiple patchy lesions, mass-like lesions, or multiple nodules (8-10). These findings can easily be mistaken for other respiratory diseases, such as pneumonia or advanced lung cancer. Due to the similarity of symptoms and CT findings with pneumonia, many patients with pulmonary ENKTCL initially receive empirical antibiotic treatment, which fails to improve their condition and instead leads to rapid deterioration and a delay in diagnosis (11-13). Currently, research on pulmonary ENKTCL is limited, with the largest study to date having included only 8 cases and conducted by pathologists to examine the clinical and pathological features of the disease (5). The CT findings and dynamic imaging changes of pulmonary ENKTCL have not been thoroughly investigated. To the best of our knowledge, this is the first large-scale and comprehensive study that investigates the clinical and CT features of patients diagnosed with primary pulmonary ENKTCL and secondary pulmonary ENKTCL.
This study was conducted to provide a comprehensive understanding of pulmonary ENKTCL by summarizing data from 23 patients. We focused on the clinical features, laboratory findings, CT presentations, CT dynamic changes, treatment strategies, and survival outcomes of these patients, with the goal of facilitating an accurate and timely diagnosis of this disease. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-365/rc).
Methods
Study population
We reviewed the medical records of patients with primary pulmonary ENKTCL and secondary pulmonary ENKTCL at our institution between January 2010 and January 2023. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Sichuan University. The requirement to obtain individual consent for this analysis was waived due to the retrospective nature of the study. All participant information was anonymized and de-identified before analysis. To be included in the study, patients had to meet the following criteria: (I) confirmed histopathological diagnosis of ENKTCL on the pathology review; (II) having undergone CT scans with adequate image quality for analysis. We did not include other related hematological malignancies, such as NK leukemia, chronic lymphoproliferative disorder of NK cells, peripheral T-cell lymphoma, and other leukemias and lymphomas analysis.
We conducted a retrospective cohort study of ENKTCL, nasal type, at West China Hospital from January 2010 to January 2023. Out of 2,677 identified pathology reports, we excluded 340 duplicates, resulting in 2,337 unique cases. Among these, 27 cases had primary or secondary pulmonary ENKTCL. Due to the absence of chest CT images, 4 cases were excluded, resulting in a final cohort of 23 patients (20 males and 3 females) for further analysis. The process of patient inclusion is depicted in Figure S1.
Clinical information from the records were collected, including patient age, symptoms, disease duration, and laboratory test results for lactate dehydrogenase (LDH), white blood cell count (WBC), hemoglobin (HGB), and platelets (PLT). The reference ranges for these parameters at our institution were: LDH: 120–250 IU/L, WBC: (3.5–9.5)×109/L, HGB: 130–175 g/L, and PLT: [100–300]×109/L.
Definition of primary and secondary pulmonary ENKTCL
Eligible cases were then divided into 2 categories: primary and secondary pulmonary ENKTCL. A diagnosis of primary pulmonary ENKTCL (PP-ENKTCL) was made when the lung was the first and only site of involvement and there was no prior history of ENKTCL. Secondary pulmonary ENKTCL (SP-ENKTCL) was diagnosed when the patient had a prior history of ENKTCL or when the initial site of ENKTCL was in another area of the body. SP-ENKTCL was classified as relapsed disease or concurrent disease based on the timing of the diagnosis of lung involvement. Specifically, if lung involvement is detected during the first-line or salvage chemotherapy for relapsed systemic disease or during the patient's remission from systemic disease, it is categorized as relapsed disease. In contrast, if the diagnosis of lung involvement is made at the time of initial ENKTCL diagnosis or shortly thereafter, it is classified as concurrent disease (14). Pulmonary involvement is indicated by high uptake of fluorodeoxyglucose (FDG) in the lungs as observed on a PET/CT scan (15).
CT image acquisition for patients in our institution
The CT acquisition protocol used for patients in our institution involved a variety of scanner models, including a 64 multi-detector CT scanner (Philips Brilliance; Philips Healthcare, Amsterdam, Netherlands) with a 120 kV voltage and collimator width of 64×0.5 mm; GE Medical Systems Revolution CT (GE Healthcare, Chicago, IL, USA) with a 120 kV voltage and collimator width of 80×0.625 mm; UIH uCT 780 (United Imaging, Shanghai, China) with a 120 kV voltage and collimator width of 40×0.5 mm. Out of the total patient cohort, 12 patients underwent additional contrast-enhanced examinations. After the unenhanced scans were obtained, a contrast agent (Omnipaque; GE Healthcare) was administered at a dose of 1 mL/kg of body weight for these patients.
The CT images were re-evaluated by 2 experienced radiologists, and the following characteristics were analyzed: location (left, right, or bilateral) of lesions, presence of mediastinal and axillary lymphadenopathy, and pleural effusion (absent, left, right, bilateral). The “floating vessels sign” and “halo sign” were also noted. Floating vessels sign indicates a large vessel encased by a mass without vascular involvement (16). The halo sign refers to a rim of ground glass opacity (GGO) surrounding a pulmonary nodule or mass (8).
The CT findings of pulmonary ENKTCL can be categorized into 5 types based on previous cases: Type 1 presents as bilateral pneumonia-like lesions with multiple patchy areas and clear or ground glass edges (10); Type 2 appears as mass-like lesions resembling bronchogenic carcinoma (9); Type 3 displays solitary or multiple nodules in both lungs; Type 4 presents as consolidation lesions (17); and Type 5, mixed lesions, which show a combination of nodules, GGOs, and consolidation in both lungs (13). Some patients have unique CT findings that cannot be categorized, and they are classified based on the predominant CT feature.
Histopathology assessment and immunosuppression for patients in our institution
A meticulous review was undertaken of pathology reports, consolidating each patient's histopathological evaluation and immunosuppression status. Key characteristics of histomorphological examination encompassed the size and growth pattern of neoplastic cells, geographic necrosis, and the inflammatory response. As part of this standard procedure, formalin-fixed, paraffin-embedded tissue sections of pulmonary ENKTCL were subjected to immunohistochemistry using a panel of monoclonal antibodies, including CD3, CD56, GranzymeB, TIA-1, CD20, CD30, and Ki-67. The staining was processed using an automated immunostainer, and both positive and negative control sections were utilized for accurate results. Furthermore, the presence of EBV infection was evaluated using in situ hybridization (ISH) with EBV-encoded small RNA (EBER) PNA probes. TCR-γ gene rearrangement was also assessed using polymerase chain reaction (PCR) based on the BIOMED-2 protocol. The resultant PCR products were then evaluated via heteroduplex analysis to determine monoclonality or polyclonality. All of these procedures are consistent with our institutional protocol and were applied uniformly across all cases in this study.
Treatment and outcome
Treatments received both prior to and after diagnosis were documented. Survival time was determined from time of diagnosis of pulmonary ENKTCL to death of any cause. Patient follow-up data were retrospectively collected from available medical records, including out-patient visits, and when necessary, through telephone interviews.
Statistical analysis
Overall survival (OS) was calculated from the date of diagnosis to the date of death or the date of last follow-up (1 March 2023). Survival analyses were estimated using the Kaplan–Meier method, and the log-rank tests were employed to compare survival curves between groups. Due to the small sample size, interpretation of clinical characteristics, treatments, and outcomes is descriptive.
Results
Baseline characteristics
A total of 23 patients, consisting of 20 males and 3 females, were included in the final analysis. The mean age of these patients was 40.39 years with a range of 18 to 58 years. The duration of symptoms ranged from 7 days to 3 years. The patients were divided into primary (n=9) and secondary (n=14) pulmonary ENKTCL (as shown in Table 1). Elevated LDH was observed in 20 (86.9%) patients.
Table 1
| Case | Disease pattern | Sex | Age (years) | Symptoms | DOS (months) | EBV-DNA | HGB (g/L) | WBC (109/L) | PLT (109/L) | LDH (IU/L) | Treatment | Survival time (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | M | 57 | Repeated fever, cough, hemoptysis | 24 | Unknown | 134 | 4.54 | 128 | 591 | AT, chemotherapy | 1 |
| 2 | P | M | 49 | Fever, cough | 1 | Unknown | 131 | 2.97 | 96 | 375 | AT | 7 |
| 3 | P | M | 33 | Fever | 3 | Unknown | 122 | 2.96 | 167 | Unknown | Unknown | Lost |
| 4 | P | M | 46 | Fever | 5 | 1.73E+04 | 128 | 4.95 | 279 | 188 | AT, chemotherapy | Alive (7 years) |
| 5 | P | M | 44 | Fever, cough | 12 | 1.79E+04 | 139 | 3.36 | 134 | 430 | AT | 1 |
| 6 | P | M | 31 | Fever, cough, sweaty | 2 | Unknown | 84 | 6.45 | 214 | 298 | AT | 1 |
| 7 | P | M | 45 | Fever | 2 | 2.11E+03 | 79 | 5.32 | 160 | 448 | AT | 1 |
| 8 | P | F | 45 | Shortness of breath, edema of both lower limbs | 6 | 1.19E+04 | 68 | 0.73 | 214 | 264 | AT, chemotherapy | 8 |
| 9 | P | M | 51 | Right upper limb pain | 1 | 1.24E+06 | 109 | 2.53 | 201 | 550 | ST | 4 |
| 10 | S, concurrent | M | 24 | Fever, abdominal pain | 1 week | 1.42E+05 | 129 | 9.9 | 35 | 260 | AT | 3 |
| 11 | S, concurrent | M | 19 | Fever, cough | 2 | Unknown | 95 | 0.9 | 69 | 437 | AT | 1 |
| 12 | S, relapse | M | 46 | 0.5 years ENKTCL history | NA | 7.21E+03 | 116 | 3.64 | 174 | 377 | Chemotherapy | 12 |
| 13 | S, relapse | M | 35 | 4 years ENKTCL history | NA | 2.36E+03 | 140 | 3.12 | 133 | 242 | AT, chemotherapy | 4 |
| 14 | S, concurrent | M | 53 | Repeated fever, cutaneous carbuncle of thigh | 3 | 1.07E+05 | 82 | 2.61 | 55 | 645 | AT | Lost |
| 15 | S, concurrent | M | 37 | Repeated fever, hematochezia | 1.5 | 3.27E+0.3 | 70 | 4.73 | 219 | 322 | AT, chemotherapy | 5 |
| 16 | S, concurrent | M | 18 | Sore throat | 1 | 1.35E+05 | 121 | 2.93 | 125 | 976 | AT | 1 |
| 17 | S, concurrent | F | 45 | Repeated fever, cough | 2 | 8.22E+05 | 93 | 2.46 | 159 | 700 | AT, chemotherapy | Alive (1.5years) |
| 18 | S, concurrent | F | 32 | Repeated fever, cough, dyspnea | 5 | Unknown | 113 | 1.95 | 149 | 814 | AT | 1 |
| 19 | S, concurrent | M | 58 | Nasal obstruction, fever | 3 | 1.29E+04 | 130 | 4.53 | 213 | 712 | AT | 1 |
| 20 | S, concurrent | M | 28 | Fever, chilly | 1 | 3.20E+04 | 98 | 0.95 | 42 | 944 | AT, chemotherapy | 2 |
| 21 | S, concurrent | M | 41 | Nasal obstruction, night sweat | 36 | Negative | 145 | 3.23 | 142 | 384 | AT, chemotherapy | Alive (2.3years) |
| 22 | S, concurrent | M | 54 | Poor appetite | 6 | 1.52E+05 | 119 | 2.72 | 232 | 336 | Chemotherapy, radiotherapy | Alive (1 years) |
| 23 | S, relapse | M | 38 | 3 years ENKTCL history | NA | <50 | 171 | 5.73 | 256 | 189 | Chemotherapy | Alive (4 m) |
The references range for these parameters at our institution were: LDH: 120–250 IU/L, WBC: (3.5–9.5)×109/L, HGB: 130–175 g/L, and PLT: [100–300]×109/L. P, primary, S, secondary; M, male; F, female; DOS, duration of symptoms; EBV, Epstein-Barr virus; HGB, hemoglobin; WBC, white blood cell count; PLT, blood platelet; LDH, lactate dehydrogenase; AT, antimicrobial treatment; ST, supportive treatment; NA, not applicable.
Morphologic and immunohistochemical findings
The neoplastic cells exhibited a wide size range, from small to large, or of a mixed-composition type. These cells showed widespread infiltration, causing partial damage to the involved lung sites. The observed growth patterns were angiocentric and anti-invasive. Patients presented a varied mix of neoplastic cells, each accompanied by different inflammatory responses. Lymphocytes and plasma cells were the most commonly observed inflammatory cells. In terms of immunostaining, every case was positive for CD3 and EBER, whereas no case showed positive staining for CD20. Of the patients included in our study, 3 were negative for CD56, and the rest were positive. For the cytotoxic granules (granzyme B and TIA-1), we observed that 20 out of the total cases were positive for at least 1 of them. Out of the 13 individuals tested for CD30, 8 were positive. These findings are summarized in Figure S2.
CT features of the 23 patients with pulmonary ENKTCL
In our study, 23 patients with pulmonary ENKTC were scanned by CT, including 9 primary cases (cases 1–9) and 14 secondary cases (cases 10–23). Among the primary cases, 1 had pleural involvement characterized by thickened pleura and significant pleural effusion (case 8). Among the secondary cases, 1 presented with bronchial stenosis (case 23). The remaining 21 cases, spanning both primary and secondary, had lung lesions with several types of involvement, which we will detail next in order of frequency: solitary or multiple nodules (7/23, 30.4%), mixed type (6/23, 26.1%), consolidation (2/23, 8.7%), mass-like (4/23, 17.4%), and multiple patchy (2/23, 8.7%) (as shown in Figure 1). Most of the involvement was bilateral. The borders of the lesions were usually not clear, with a halo sign observed in 18 cases (18/23, 78.3%). Of the 23 patients, pleural effusion was present in 13 patients (bilateral: 6 cases; right: 5 cases; left: 2 cases), and 12 patients had mediastinal or axillary lymphadenopathy (as summarized in Table 2).

Table 2
| Case | Laterality | CT patterns | Mediastinal lymphadenopathy | Pleural effusion | Halo sign | Floating vessels sign | Misdiagnosis |
|---|---|---|---|---|---|---|---|
| 1 | Left | Consolidation | Yes | Left | Yes | Yes | Lung cancer |
| 2 | Bilateral | Mixed lesions | No | Left | Yes | Yes | Pneumonia |
| 3 | Bilateral | Multiple nodules | No | Right | Yes | Yes | Pneumonia |
| 4 | Right | Mass-like | No | No | Yes | Yes | Lung cancer |
| 5 | Bilateral | Mixed lesions | Yes | Bilateral | Yes | Yes | Pneumonia (fungal?) |
| 6 | Bilateral | Mixed lesions | Yes | No | Yes | Unknown | Pneumonia |
| 7 | Bilateral | Multiple patchy | No | No | Yes | Unknown | Pneumonia |
| 8 | Right | Thickening of the pleura | Yes | Bilateral | No | Unknown | Tuberculous pleurisy |
| 9 | Right | Mass-like | Yes | No | Yes | No | Lung cancer |
| 10 | Bilateral | Multiple nodules | Yes | Right | Yes | Yes | Pneumonia |
| 11 | Bilateral | Mixed lesions | No | Right | Yes | Unknown | Pneumonia |
| 12 | Left | Mass-like | No | No | No | Unknown | Lung cancer |
| 13 | Bilateral | Mixed lesions | No | No | Yes | Yes | Pneumonia (fungal?) |
| 14 | Bilateral | Multiple nodules | No | Bilateral | Yes | Unknown | Pneumonia |
| 15 | Bilateral | Multiple nodules | No | No | Yes | Yes | Pneumonia |
| 16 | Bilateral | Mixed lesions | Yes | Bilateral | Yes | Yes | Pneumonia |
| 17 | Bilateral | Multiple nodules | No | No | Yes | Unknown | Pneumonia |
| 18 | Bilateral | Multiple patchy | Yes | Bilateral | Yes | Unknown | Pneumonia |
| 19 | Right | Consolidation | Yes | Bilateral | Yes | Yes | Lung cancer |
| 20 | Bilateral | Multiple nodules | Yes | Right | Yes | Unknown | Pneumonia |
| 21 | Left | Nodule | Yes | No | Yes | Unknown | No |
| 22 | Right | Mass-like | Yes | Right | No | Yes | Lung cancer |
| 23 | Right | bronchial stenosis | Yes | No | No | Unknown | No |
CT, computed tomography.
Out of the 23 patients who underwent CT examinations, 12 patients received contrast-enhanced scans. The vessel floating sign was observed in the lesions of 10 (10/12, 83.3%) patients (as show in Figure 2).
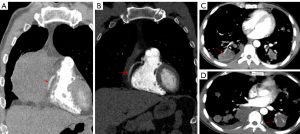
We compared the CT features between primary and secondary pulmonary ENKTCL. In the patients with PP-ENKTCL, the most common CT presentation was mixed lesions type, with 3 cases showing this pattern. Mass-like appearance was seen in 2 cases, consolidation in 1 case, multiple patchy opacity in 1 case, pleural thickening in 1 case, and multiple nodules in 1 case. Multiple nodules were the most common CT presentation in SP-ENKTCL, with 6 cases showing this pattern, followed by mixed lesions in 3 cases, mass-like lesion in 2 cases, consolidation in 1 case, multiple patchy in 1 case, bronchial stenosis in 1 case.
CT follow-up
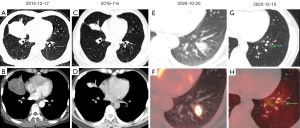
Of the 23 patients in this study, 15 had follow-up CT scans performed. During the follow-up period, 7 patients experienced an increase in the number or size of lung lesions. Among these 7 patients, 6 were treated with anti-infective therapy during the follow-up, whereas 1 underwent chemotherapy. Figure 3 showcases representative CT images illustrating the rapid progression of pulmonary ENKTCL in 3 patients who received antibiotic treatment.

In addition, 3 patients demonstrated improvement after undergoing chemotherapy. Of them, 2 patients displayed a reduction in lesion size (both initially presented as mass-like on CT), and 1 experienced complete disappearance of the lung lesions, as depicted in Figure 4. In contrast, the remaining 5 cases did not exhibit any significant changes throughout the follow-up period.
Diagnostic challenges, treatment options, and survival outcomes
In our series of 23 cases, initial diagnoses were incorrect for 21 patients: 14 were first diagnosed with pneumonia, 6 with lung cancer, and 1 with tuberculosis. Timely use of PET/CT or fiberoptic bronchoscopy led to accurate diagnoses for only 2 patients. Initial treatment for most patients (19 cases) involved antimicrobial medication, whereas 11 patients underwent chemotherapy (with 1 additionally receiving radiotherapy) following their final diagnosis. Treatment choices varied among patients: one opted for supportive care, another refused treatment, and others undertook different chemotherapy regimens such as GLIDE (5 patients), P-Gemox (4 patients), SMILE (1 patient), and CHOP (1 patient). Cases 20 and 21 also received anti-programmed cell death protein 1 (PD-1) immunotherapy.
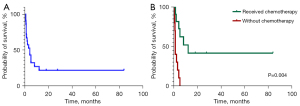
Outcomes were available for 21 cases at our institution: 16 patients had passed away, whereas 5 patients are alive and under follow-up. Of the surviving patients, 3 have been in complete remission (CR) for 1.5–7 years, with the remaining 2 still undergoing treatment. The median OS was found to be 4 months. We observed that chemotherapy typically enhanced the prognosis (Figure 5), with a median OS of 12 months in patients who received this treatment.
Discussion
In this study, we explored the clinical and CT characteristics, treatment outcomes, and prognostic factors of pulmonary ENKTCL, a rare and aggressive non-Hodgkin lymphoma. Prior literature on pulmonary ENKTCL is largely limited to case reports, leaving substantial gaps in the understanding of its prognosis and treatment. Our findings show a generally poor prognosis, with a median OS of just 1 month. Notwithstanding, a subset of patients demonstrated a more favorable prognosis. The factors such as the timely administration of chemotherapy and specific CT patterns may significantly impact the outcome of pulmonary ENKTCL.
The radiographic presentations of pulmonary ENKTCL on CT scans, similar to other types of pulmonary lymphomas, exhibit a wide range of variability. We found that secondary pulmonary ENKTCL are more likely to manifest as multiple nodules, whereas primary pulmonary ENKTCL tend to present as mixed lesions. These findings are partly in line with previous studies by Dong et al. and Zhang et al., who separately analyzed CT findings in primary and secondary pulmonary lymphomas. They reported that multiple nodules are a more frequent manifestation in secondary pulmonary lymphomas, while consolidation is the most common presentation in primary pulmonary lymphomas (6,15). However, it is important to note that their analyses included a limited number of ENKTCL cases (4 patients).
Diagnosing pulmonary ENKTCL can be challenging due to the absence of specific symptoms and the variety of CT findings. Therefore, differentiation from other conditions such as pneumonia and lung cancer are necessary (9,18-20). The presence of pancytopenia and elevated levels of LDH, which are common laboratory findings in ENKTCL, can aid in the diagnostic process (21). Kikuchi et al.’s findings also highlight the potential value of soluble interleukin-2 receptor (sIL-2R) in pulmonary lymphoma (22). Moreover, patients may show rapid progression of lung lesions and a decline in their clinical condition after receiving antibiotics, indicating that the cause is not an infection. Furthermore, the “floating vessels sign” observed on enhanced CT scans may serve as a useful clue in diagnosing pulmonary ENKTCL. This sign is thought to result from lymphoma originating in the interstitial tissue of the organ and spreading along the interstitial space, preserving the anatomy of the vessels. Lymphoma lesions are primarily composed of single cell proliferation and appear as slightly lower density uniformities on CT scans, with the vessels visible as the “floating vessels sign” within this uniform background (16). In contrast, lung cancer typically invades blood vessels, causing rough blood vessel walls and unclear lumens (23). Although a definitive diagnosis may be difficult, it is important to consider the possibility of pulmonary lymphoma by considering all the laboratory test results, the failure of antibiotics treatment, and the CT findings. While PET/CT may not be specific in diagnosing distinct types of lymphoma, it remains an important diagnostic tool for patients with pulmonary ENKTCL (24). ENKTCL can affect any organ or tissue and some involved sites may be hidden; PET/CT is highly effective in identifying the affected areas (25).
The prognosis of pulmonary ENKTCL is poor, much worse than that of other types of aggressive lymphomas such as peripheral T-cell lymphoma and diffuse large B-cell lymphoma (6). Although the chemotherapeutic regimen was not standardized, our study indicates that administrating chemotherapy improved the prognosis, with a median OS of 12 months in patients who received this treatment.
We found that patients with a poorer prognosis frequently exhibited consolidation and mixed lesion patterns, whereas nodular lesions were more prevalent in those with a better prognosis. We propose a hypothesis that pulmonary ENKTCL may initially manifest as nodule/mass-like lesions during its early phase, progressing to consolidation or mixed type lesions in the later stages. This progression can be tracked via CT follow-up scans, as depicted in Figure 3 of our study. Among our case series, 3 patients who achieved CR ranging from 1.5 to 7 years, presented CT findings of a mass-like lesion, a solitary nodule, and multiple small nodules, respectively. These observations underscore the crucial role of early detection and immediate treatment in enhancing the prognosis of pulmonary ENKTCL, a point also highlighted by Yabushita et al., where early diagnosis enabled a patient to maintain continuous CR for 1-year post-diagnosis (26). Nevertheless, a more comprehensive understanding of this relationship necessitates further research.
Our study has some limitations. As a retrospective study, not all patients underwent consistent diagnostic or follow-up procedures such as enhanced CT scans, CT follow-ups, PET scans, or uniform laboratory tests. This variability made the comprehensive analysis of pulmonary ENKTCL challenging, potentially influencing the outcomes. Likewise, the treatment approaches, particularly chemotherapy, also varied considerably among patients, which may have influenced prognosis. Future research should aim to include larger sample sizes and more standardized analyses to validate and build upon our results.
Conclusions
Pulmonary ENKTCL is a rare disease with a high degree of invasiveness. Laboratory tests, the failure of antibiotics treatment, as well as the “floating vessels sign” observed on enhanced CT scans, may serve as clues in the diagnosis. Survival among patients with pulmonary ENKTCL is poor, but timely diagnosis and chemotherapy may yield long-term survival for some patients. This study fills a critical gap in our understanding of pulmonary ENKTCL, paving the way for the development of more effective diagnostic and treatment strategies to improve patient outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-365/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-365/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by Institutional Review Board of Sichuan University. The requirement to obtain individual consent for this analysis was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang H, Fu BB, Gale RP, Liang Y NK. -/T-cell lymphomas. Leukemia 2021;35:2460-8. [Crossref] [PubMed]
- Asano N, Kato S, Nakamura S. Epstein-Barr virus-associated natural killer/T-cell lymphomas. Best Pract Res Clin Haematol 2013;26:15-21. [Crossref] [PubMed]
- Tse E, Fox CP, Glover A, Yoon SE, Kim WS, Kwong YL. Extranodal natural killer/T-cell lymphoma: An overview on pathology and clinical management. Semin Hematol 2022;59:198-209. [Crossref] [PubMed]
- Laohaburanakit P, Hardin KA. NK/T cell lymphoma of the lung: a case report and review of literature. Thorax 2006;61:267-70. [Crossref] [PubMed]
- Ding W, Wang J, Zhao S, Yang Q, Sun H, Yan J, Gao L, Yao W, Zhang W, Liu W. Clinicopathological study of pulmonary extranodal nature killer/T-cell lymphoma, nasal type and literature review. Pathol Res Pract 2015;211:544-9. [Crossref] [PubMed]
- Zhang MC, Zhou M, Song Q, Wang S, Shi Q, Wang L, Yan FH, Qu JM, Zhao WL. Clinical features and outcomes of pulmonary lymphoma: A single center experience of 180 cases. Lung Cancer 2019;132:39-44. [Crossref] [PubMed]
- Chien CC, Lee HS, Lin MH, Hsieh PP. Primary extranodal natural killer/T-cell lymphoma of bronchus and lung: A case report and review of literature. Thorac Cancer 2016;7:140-4. [Crossref] [PubMed]
- Fei W, Xiaohong W, Hong Z, Bei H. Pulmonary Extranodal Natural Killer/T-Cell Lymphoma (Nasal Type): A Case Report and Radiological Image Review. Medicine (Baltimore) 2015;94:e1527. [Crossref] [PubMed]
- Liu CH, Wang HH, Perng CL, Peng CK, Chian CF, Shen CH. Primary extranodal NK/T-cell lymphoma of the lung: Mimicking bronchogenic carcinoma. Thorac Cancer 2014;5:93-6. [Crossref] [PubMed]
- Wang W, Li ZT, Cui NN, Wang GB, Fu SQ. Acute respiratory distress syndrome emerging after surgical debridement in a patient with extranodal natural killer/T cell lymphoma. BMC Pulm Med 2021;21:27. [Crossref] [PubMed]
- Papiris SA, Kagouridis K, Kolilekas L, Triantafillidou C, Tsangaris I, Manali ED. Pirfenidone treatment in idiopathic pulmonary fibrosis: too much of a great expectation? Eur Respir J 2012;40:794-5. [Crossref] [PubMed]
- Lee S, Shin B, Yoon H, Lee JY, Chon GR. A case of primary pulmonary NK/T cell lymphoma presenting as pneumonia. Respir Med Case Rep 2016;17:1-4. [Crossref] [PubMed]
- Qiu Y, Hou J, Hao D, Zhang D. Primary pulmonary NK/T-cell lymphoma: A case report and literature review. Mol Clin Oncol 2018;8:753-6. [Crossref] [PubMed]
- Nevel KS, Pentsova E, Daras M. Clinical presentation, treatment, and outcomes of patients with central nervous system involvement in extranodal natural killer/T-cell lymphoma. Leuk Lymphoma 2019;60:1677-84. [Crossref] [PubMed]
- Dong Y, Zeng M, Zhang B, Han L, Liu E, Lian Z, Liu J, Liang C, Zhang S. Significance of imaging and clinical features in the differentiation between primary and secondary pulmonary lymphoma. Oncol Lett 2017;14:6224-30. [Crossref] [PubMed]
- Yoshihara S, Sugimoto Y, Matsunaga M, Suzuki S, Tanioka F. Coronary vessel floating sign in cardiac diffuse large B-cell lymphoma. Eur Heart J Cardiovasc Imaging 2020;21:233. [Crossref] [PubMed]
- Wang Y, Wang Z, Wu C, Zhao X, Ji N, Huang M. Primary pulmonary extranodal NK/T-cell lymphoma: a case report and literature review. Transl Cancer Res 2020;9:7359-65. [Crossref] [PubMed]
- Lee BH, Kim SY, Kim MY, Hwang YJ, Han YH, Seo JW, Kim YH, Cha SJ, Hur G. CT of nasal-type T/NK cell lymphoma in the lung. J Thorac Imaging 2006;21:37-9. [Crossref] [PubMed]
- Song M, Kim JY, Choi JS, Yoon B, Kim M, Kim SJ, Kim SY. Primary Pulmonary Extranodal Natural Killer/T-cell Lymphoma, Nasal Type Presenting as Diffuse Ground Glass Opacities: a Case Report. J Korean Med Sci 2017;32:1727-30. [Crossref] [PubMed]
- Davis BW, Beasley MB, Dua S. Primary Pulmonary Natural Killer/T-Cell Lymphoma Presenting as Nonresolving Pneumonia. Chest 2010;138:18A. [Crossref]
- Na II, Kang HJ, Park YH, Lee SS, Yoo HJ, Choe DH, Ryoo BY, Yang SH. Prognostic factors for classifying extranodal NK/T cell lymphoma, nasal type, as lymphoid neoplasia. Eur J Haematol 2007;79:1-7. [Crossref] [PubMed]
- Kikuchi R, Ishiwari M, Takoi H, Kono Y, Yoshimura A, Abe S. Pulmonary intravascular lymphoma mimicking hypersensitivity pneumonitis. Pulmonology 2020;26:409-12. [Crossref] [PubMed]
- Laurent F, Montaudon M, Corneloup O. AMECT and MRI of Lung Cancer. Respiration 2006;73:133-42. [Crossref] [PubMed]
- Peng Y, Qi W, Luo Z, Zeng Q, Huang Y, Wang Y, Sharma A, Schmidt-Wolf IGH, Liao F. Role of 18F-FDG PET/CT in patients affected by pulmonary primary lymphoma. Front Oncol 2022;12:973109. [Crossref] [PubMed]
- El-Galaly TC, Villa D, Gormsen LC, Baech J, Lo A, Cheah CY. FDG-PET/CT in the management of lymphomas: current status and future directions. J Intern Med 2018;284:358-76. [Crossref] [PubMed]
- Yabushita T, Yoshioka S, Furumiya T, Nakamura M, Yamashita D, Imai Y, Ishikawa T. The impact of early diagnosis on the prognosis of extranodal NK/T-cell lymphoma with massive lung involvement: a case report. BMC Pulm Med 2019;19:48. [Crossref] [PubMed]


