
Treatment response assessment to chemotherapy with bevacizumab for colorectal liver metastasis by contrast-enhanced ultrasound
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide. Metastases would happen in around 40–70% CRC patients, with liver being the most common metastatic site (1). The 5-year survival rate for patients with colorectal liver metastasis (CRLM) ranges from 3% to 47%, and hepatectomy is the most effective therapy for these patients (2,3). However, up to 75% CRLM patients cannot receive radical treatment due to its multiplicity or large size at their presentation (3). Cytotoxic and anti-angiogenic target therapy is therefore commonly utilized nowadays (4-7). According to the National Comprehensive Cancer Network (NCCN) guidelines in 2013, FOLFOX (5-fluorouracil, levofolinate, and oxaliplatin) plus bevacizumab (Bev), or FOLFIRI (5-fluorouracil, levofolinate, and irinotecan) plus Bev has been selected as the standard first-line chemotherapy for CRLM (8).
The treatment response assessment is essential and important for the management of CRLM due to the adverse effects and high cost of target therapy. For liver malignancies, several criteria have been carried out in clinical working, including Response Evaluation Criteria in Solid Tumor (RECIST) (9), European Association for the Study of the Liver (EASL) (10) and modified RECIST (mRECIST) (11), with the last being the most commonly used criteria nowadays.
Currently, different imaging techniques, including dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), DCE-computed tomography (CT), and positron emission tomography (PET)/CT have been widely investigated for response assessment in CRLM under treatment by measuring changes on size, anatomies or functions (12-15). Though contrast-enhanced CT (CECT) and MRI have been deemed as the standard modality for evaluation after chemotherapy for CRLM, no reliable and widely acceptable imaging biomarkers have been established till now. Furthermore, the radiation and potential nephrotoxicity restricted their application under certain circumstances, especially in those with chronic renal insufficiency. Moreover, rare study has ever explored the change of blood perfusion in the assessment of treatment response (13-15).
Contrast-enhanced ultrasound (CEUS) is the exclusive imaging technique that has a pure intra-vascular contrast agent, which effectively disregards diffusion and leakage into the mesenchyma (16). Promising results have been achieved from studies on CEUS perfusion parameters in revealing the change of vascularity in different tumors under treatment, such as gastrointestinal stromal tumor, renal cell carcinoma, and hepatocellular carcinoma (HCC) (17). Bev, a monoclonal antibody against vascular endothelial growth factor (VEGF), aims to modify the vascularity and microcirculation of tumor (4); therefore, CEUS perfusion parameters have been hypothesized to be potential indicators for positive clinical response to chemotherapy with Bev in patients with CRLM (12).
The purpose of this study was to evaluate the feasibility of CEUS perfusion parameters in assessing treatment response of CRLM to chemotherapy with Bev, using mRECIST as the standard reference (9,11). We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1027/rc).
Methods
Study subjects
This prospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Zhongshan Hospital, Fudan University approved the study (No. B2021-347R), and written informed consent was received from all patients. From March 2021 to May 2022, consecutive patients with CRLM who underwent chemotherapy with Bev in our institution were enrolled in this prospective study. Inclusion criteria were: (I) diagnosed as CRLM for the first time, no matter whether the primary cancer has been resected; (II) patients underwent chemotherapy plus Bev treatment, without other systemic or local treatment before; (III) no radiological evidence of gross vascular or biliary invasion; (IV) adequate kidney and liver function; and (V) size of target tumor ≥1.5 cm, necrotic spot (cystic portion) ≤50% of total tumor volume on gray-scale ultrasound measurement. Exclusion criteria were: (I) patients without post-treatment CEUS examination (n=6); (II) poor quality of CEUS image for quantitative analysis (i.e., fragile breath, and out-of-plane movement, incomplete CEUS videos) (n=3); and (III) invalid response evaluation by mRECIST or disagreement of the response evaluation between two independent radiologists (n=2). The included patients were divided into two cohorts in chronological order: (I) cohort I: development cohort (n=89, between March 2021 and December 2021) and (II) cohort II: validation cohort (n=26, between January 2022 and May 2022).
CEUS examination and data acquisition
All ultrasound examinations were performed by two sonologists with 12- and 14-year CEUS experience, independently, using iU22 or Epiq7 system (Philips Healthcare, Bothell, WA, USA) with a 1–5 MHz transducer.
In our institute, the CEUS examination of liver lesion has a routine procedure. First, B-mode examination was performed to identify the target lesion for investigation, and scanning plane covering the largest tumor dimension and adjacent liver parenchyma. Since only one lesion per patient can be analyzed per injection of contrast agent, for patients with multiple tumors, only one target lesion was selected. The criteria for the target lesion selection were as follows: (I) the largest one; (II) lesion with best acoustic window; and (III) lesion adjacent to anatomical marks (gallbladder, hepatic vein, portal vein, etc.) for precisely position the target lesion during follow-up. For response assessment, the target lesion for CEUS examination must keep the same during follow-up.
For CEUS examination, 2.0 mL of Sonovue (Bracco, Italy) was antecubital injected as a bolus, followed by a 5 mL flush of 0.9% NaCl solution. During CEUS examination, the patient was asked to slightly half-fill breath and the probe was held steadily to minimize the influence of motion. System settings were fixed during CEUS follow-up: gain (75%), mechanical index (0.06), and dynamic range (70 dB), frame rate (12 fps), depth of image (14 cm), and one focus below the target lesion. The whole process of CEUS examination lasted at least 3 min, and was recorded in real time as DICOM format on the hard disc for off-line analysis. Each patient underwent CEUS examinations for at least 2 times with the same US scanner: (I) before treatment, with the interval between CEUS and treatment ≤3 days; (II) 2-month after treatment, at which point 4 cycles of treatment ended, and the interval between CEUS and CECT (mRECIST modality) ≤3 days.
CEUS perfusion parameter analysis
SonoLiver (Bracco Research SA, Geneva, Switzerland and TomTec Imaging System, Unterschleissheim, Germany) was adopted as the quantitative analysis software (18). The perfusion parameter analysis was performed by another sonologist with 10-year experience in liver CEUS interpretation who was blinded to the clinical information of the subjects.
The quantitative analysis included three consecutive steps: First, out-of-plane images and images preceding contrast agent arrival in the hepatic arterial (set as time “0”) were excluded from processing. Second, a representative image was selected on which the lesion was well delineated, generally at peak enhancement. This image served as reference frame for motion compensation, which was equipped with SonoLiver to automatically minimize the influence of slight breath on the quantitative results. Last, two types of region of interest (ROI) were manually drawn on the reference frame, including analysis ROI and reference ROI, to generate time-intensity curves (TICs) of different ROIs, where the intensity were linearized and log-compressed. The quality of fit (QOF) between original perfusion curve and the best-fitting perfusion curve was automatically generated to insure that the model used was adequate with QOF ≥80%. If the QOF of the quantitative analysis was <80%, the case would be excluded from the study.
In present study, to explore the influence of ROI selection on perfusion parameters, three different analysis ROIs were selected for each lesion, one ROI covering the whole lesion (ROIwhole), one ROI selected on lesion periphery (ROIperipheral), and one ROI selected on internal tumor with necrotic area avoided (ROIinternal). For reference ROI (ROIreference), it was selected on the adjacent liver parenchyma at the same depth of tumor with artifacts, large vessels, calcification, and liver capsule avoided. The shape of ROIperipheral, ROIinternal, and ROIreference was round and their size ranged from 1–2 cm × 1–2 cm. While the shape and size of ROIwhole were unlimited and dependent on the target lesion (Figure 1).

Then five quantitative perfusion parameters were extracted from the TICs for tumor automatically by SonoLiver software, including: maximal intensity (IMAX; defined as the peak intensity of tumor TIC/intensity of background × 100%), area under the TIC (AUC; integration of intensity under tumor TIC), rise time (RT; time slot for the increase of intensity from 10% to 100% of the peak on tumor TIC), time-to-peak (TTP; time slot from the emergence of contrast agent to peak intensity on tumor TIC), and mean transit time (MTT; time slot for the decrease in intensity from peak to 50% on tumor TIC). The reduction and ratio of a parameter pre- and post-treatment were further calculated. Reduction of a parameter (∆par.) was calculated as the value for pre-treatment minus that for post-treatment. And the ratio of a parameter was defined as post-treatment value/pre-treatment value.
Treatment response evaluation
To evaluate the treatment response, each patient underwent CECT scans both pre- and 2 months post-treatment to generate mRECIST grade as standard reference. The interval between CEUS and CECT was within 3 days.
According to mRECIST, target lesion responses were graded as follows: complete response (CR; disappearance of any intra-tumoral arterial enhancement), partial response (PR; at least a 30% decrease in the sum of diameters of viable target lesions, taking as reference the baseline sum of the diameters of viable target lesions recorded before treatment), progressive disease (PD; an increase of at least 20% in the sum of the diameters of viable target lesions), and stable disease (SD; all other variations) (15).
In present study, patients with CR or PR were defined as responders, while patients with SD or PD were non-responders. Two independent radiologists, blinded to the clinical information, independently performed the grading. Once disagreement appeared, the subject would be excluded from this study.
Statistical analysis
MedCal (10.4.7.0, Frank Schoonjans, Belgium) was utilized to perform all the statistical analysis. Mann-Whitney test was applied on each perfusion parameter to study the difference between pre- and post-treatment. Difference was considered significant with P<0.05 (two-tailed). Box-and-whisker plot was applied to illustrate the distributions of reduction and ratio of perfusion parameter. Area under the receiver operating characteristic curve (AUROC) analysis was applied to evaluate the performance of perfusion parameter in assessing treatment response in both development and validation cohort. The AUROC was classified as low (0.50–0.70), moderate (0.71–0.90), and high (0.91–1.00). Accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated with cut-offs that maximize the sum of sensitivity and specificity. The influence of ROI selection on the performance of perfusion parameters in assessing treatment response was further studied by comparing the AUROCs of ROIwhole, ROIperipheral, and ROIinternal, respectively.
Results
Patient characteristics
Figure 2 is the flow chart of the patient recruitment. Thus, the final study subjects were consisted of 115 patients (age 45±16 years; male 78, female 37), including 87 received FOLFOX plus Bev and 28 received FOLFIRI plus Bev. No significant difference in patient characteristics was found between the two cohorts (P>0.10 for all) (Table 1).
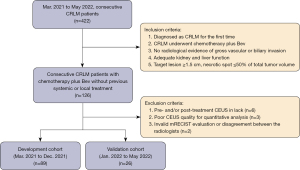
Table 1
| Variables | Development cohort (n=89) | Validation cohort (n=26) | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | PR | SD | PD | Overall | PR | SD | PD | |||
| General information | ||||||||||
| Total | 89 [100] | 48 [54] | 30 [34] | 11 [12] | 26 [100] | 14 [54] | 9 [35] | 3 [11] | 0.98 | |
| Age (years) | 46±18 | 42±15 | 50±21 | 47±17 | 42±15 | 44±18 | 40±12 | 45±14 | 0.47 | |
| Male | 59 [66] | 34 [71] | 19 [63] | 6 [55] | 19 [73] | 12 [86] | 7 [78] | 0 [0] | 0.64 | |
| Location of primary tumor | 0.61 | |||||||||
| Colon | 66 [74] | 35 [73] | 23 [77] | 8 [73] | 21 [81] | 11 [79] | 7 [78] | 3 [100] | ||
| Rectum | 23 [26] | 13 [27] | 7 [23] | 3 [27] | 5 [19] | 3 [21] | 2 [22] | 0 [0] | ||
| Histology of primary tumor | 0.68 | |||||||||
| Mucinous carcinoma | 8 [9] | 0 [0] | 5 [17] | 3 [27] | 1 [4] | 0 [0] | 1 [11] | 0 [0] | ||
| Non-mucinous carcinoma | 81 [91] | 48 [100] | 25 [83] | 8 [73] | 25 [96] | 14 [100] | 8 [89] | 3 [100] | ||
| Stage at diagnosis | 0.34 | |||||||||
| III-B | 7 [8] | 5 [10] | 2 [7] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||
| III-C | 13 [15] | 7 [15] | 4 [13] | 2 [18] | 4 [15] | 2 [14] | 2 [22] | 0 [0] | ||
| IV | 69 [77] | 36 [75] | 24 [80] | 9 [82] | 22 [85] | 12 [86] | 7 [78] | 3 [100] | ||
| Size on ultrasound (cm) | 0.32 | |||||||||
| Pre-treatment | 5.3±2.1 | 6.1±2.6 | 5.1±1.6 | 4.5±2.0 | 4.6±1.7 | 4.4±2.0 | 4.8±1.6 | 5.2±1.8 | ||
| Post-treatment | 4.6±2.4 | 4.0±1.9 | 4.5±2.2 | 6.2±2.1 | 3.8±1.8 | 3.0±1.6 | 4.4±1.8 | 7.1±2.1 | ||
| Biomarkers | 0.21 | |||||||||
| CEA pre-treatment (ng/mL) | 1,431±1,035 | 1,233±882 | 1,601±1,204 | 1,359±1,120 | 1,244±918 | 1,427±1,031 | 982±773 | 1,306±984 | ||
| CEA post-treatment (ng/mL) | 320±251 | 198±113 | 486±312 | 896±437 | 352±214 | 163±120 | 398±227 | 664±417 | ||
| CA19-9 pre-treatment (U/mL) | 584±320 | 635±336 | 552±263 | 563±302 | 664±298 | 622±269 | 703±331 | 528±225 | ||
| CA19-9 post-treatment (U/mL) | 173±98 | 109±51 | 275±130 | 302±111 | 145±83 | 98±36 | 311±141 | 370±472 | ||
Data are presented as n [%] or mean ± standard deviation. PR, partial response; SD, stable disease; PD, progressive disease; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19-9.
CEUS perfusion parameters pre- vs. post-treatment
The comparisons of CEUS perfusion parameters pre- and post-treatment in development cohort are illustrated in Table 2. According to mRECIST, 48 patients were responders (48 PR), and 41 patients were non-responders (30 SD and 11 PD). In the PR group, significantly smaller post-treatment values of IMAX and AUC were observed on ROIwhole, ROIperipheral, and ROIinternal (all P<0.001). Similar finding was observed for MTT on ROIinternal (P=0.01). For other parameters, no significantly different post-treatment values were observed. In the SD group, only significantly smaller AUC value on ROIwhole was found post-treatment (P=0.03). For other parameters, comparable values were observed. In the PD group, no significantly different post-treatment values were found on all parameters from all three analysis ROIs, compared with the corresponding parameters pre-treatment.
Table 2
| Treatment response | IMAX (%) | AUC (/100) | RT (s) | TTP (s) | MTT (s) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P value | Pre | Post | P value | Pre | Post | P value | Pre | Post | P value | Pre | Post | P value | |||||
| PR (n=48) | |||||||||||||||||||
| Whole lesion | 105±71 | 33±23 | <0.001 | 65±41 | 20±12 | <0.001 | 14.4±5.6 | 15.5±5.6 | 0.28 | 15.3±6.0 | 16.6±6.3 | 0.26 | 146±90 | 178±124 | 0.16 | ||||
| Lesion periphery | 151±103 | 40±26 | <0.001 | 87±61 | 22±15 | <0.001 | 14.3±6.0 | 14.8±6.0 | 0.61 | 15.4±6.5 | 16.1±7.0 | 0.55 | 128±110 | 162±123 | 0.10 | ||||
| Internal lesion | 86±72 | 28±24 | <0.001 | 4.8±3.4 | 1.5±1.2 | <0.001 | 15.0±7.4 | 15.7±6.2 | 0.38 | 16.2±7.9 | 17.0±7.0 | 0.41 | 117±64 | 169±114 | 0.01 | ||||
| SD (n=30) | |||||||||||||||||||
| Whole lesion | 61±22 | 51±22 | 0.08 | 39±1.6 | 31±15 | 0.03 | 14.4±4.8 | 13.6±4.3 | 0.55 | 15.2±5.2 | 14.4±4.4 | 0.66 | 185±141 | 175±147 | 0.70 | ||||
| Lesion periphery | 84±37 | 68±30 | 0.09 | 48±21 | 40±18 | 0.10 | 13.3±5.5 | 12.7±4.2 | 0.67 | 14.0±5.7 | 13.4±4.4 | 0.72 | 179±169 | 151±128 | 0.72 | ||||
| Internal lesion | 88±31 | 82±26 | 0.56 | 52±18 | 49±23 | 0.65 | 10.2±3.0 | 9.9±4.1 | 0.80 | 10.4±3.1 | 10.1±4.3 | 0.85 | 280±193 | 304±202 | >0.99 | ||||
| PD (n=11) | |||||||||||||||||||
| Whole lesion | 80±34 | 73±23 | 0.85 | 48±20 | 46±22 | 0.75 | 10.8±3.2 | 10.8±3.9 | 0.80 | 11.0±3.3 | 11.0±4.0 | 0.85 | 286±201 | 353±201 | 0.65 | ||||
| Lesion periphery | 37±27 | 33±21 | 0.42 | 51±18 | 49±23 | 0.61 | 14.3±5.0 | 14.2±5.7 | 0.85 | 15.4±5.6 | 15.6±7.4 | 0.83 | 134±100 | 135±108 | 0.89 | ||||
| Internal lesion | 74±40 | 60±32 | 0.44 | 44±2.4 | 41±25 | 0.65 | 11.5±4.9 | 12.6±5.9 | 0.52 | 11.8±5.0 | 13.1±6.3 | 0.56 | 258±178 | 291±195 | 0.85 | ||||
Data are presented as mean ± standard deviation. CEUS, contrast-enhanced ultrasound; IMAX, maximal intensity; AUC, area under the time-intensity curve; RT, rise time; TTP, time-to-peak; MTT, mean transit time; PR, partial response; SD, stable disease; PD, progressive disease.
Profiles for reduction of CEUS perfusion parameters
Profiles for reduction of CEUS perfusion parameters between responders and non-responders in development cohort are provided in Table 3 and Figure 3. For IMAX of pre-treatment minus that of post-treatment (∆IMAX), significantly higher values were revealed in responders as compared to non-responders on ROIwhole (72±55 vs. 9.0±16, P<0.001), ROIperipheral (102±70 vs. 13±24, P<0.001) and ROIinternal (53±47 vs. 7.0±15, P<0.001), respectively, as illustrated in Figure 3A. For AUC of pre-treatment minus that of post-treatment (∆AUC), significantly higher values were revealed in responders on ROIwhole (45±34 vs. 6.4±12, P<0.001), ROIperipheral (64±55 vs. 7.2±13, P<0.001), and ROIinternal (33±27 vs. 4.1±9.7, P<0.001), respectively, as illustrated in Figure 3B. For MTT of pre-treatment minus that of post-treatment (∆MTT) on ROIinternal, significantly smaller value was revealed in responders as compared to non-responders (−37±87 vs. −25±112, P<0.001).
Table 3
| Variables | Responders (n=48) | Non-responders (n=41) | P value | AUROC (95% CI) |
|---|---|---|---|---|
| ∆IMAX (%) | ||||
| Whole lesion | 72±55 | 9.0±16 | <0.001 | 0.942 (0.871–0.981) |
| Lesion periphery | 102±70 | 13±24 | <0.001 | 0.939 (0.867–0.979) |
| Internal lesion | 53±47 | 7.0±15 | <0.001 | 0.898 (0.816–0.952) |
| ∆AUC (/100) | ||||
| Whole lesion | 45±34 | 6.4±12 | <0.001 | 0.905 (0.824–0.957) |
| Lesion periphery | 64±55 | 7.2±13 | <0.001 | 0.951 (0.883–0.985) |
| Internal lesion | 33±27 | 4.1±9.7 | <0.001 | 0.885 (0.800–0.943) |
| ∆MTT (s) | ||||
| Internal lesion | −37±87 | −25±112 | <0.001 | 0.601 (0.492–0.703) |
Data are presented as mean ± standard deviation. CEUS, contrast-enhanced ultrasound; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; ∆IMAX, IMAX of pre-treatment minus that of post-treatment; IMAX, maximal intensity; ∆AUC, AUC of pre-treatment minus that of post-treatment; AUC, area under the time-intensity curve; ∆MTT, MTT of pre-treatment minus that of post-treatment; MTT, mean transit time.
In discriminating responders from non-responders, poor performance was found for ∆MTT on ROIinternal with AUROC of 0.601 (0.492–0.703). While for ∆IMAX and ∆AUC, AUROCs of 0.898–0.942, and 0.885–0.951 were observed, respectively, from different analysis ROIs.
Profiles for ratio of CEUS perfusion parameters
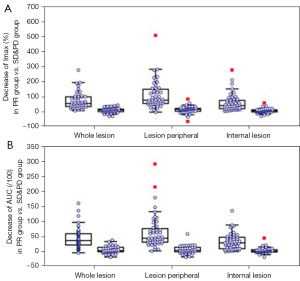
Profiles for ratio of CEUS perfusion parameter between responders and non-responders in development cohort are provided in Table 4 and Figure 4. For ratio of IMAX, significantly lower values were revealed in responders as compared to non-responders on ROIwhole (0.34±0.16 vs. 0.90±0.29, P<0.001), ROIperipheral (0.29±0.20 vs. 0.88±0.36, P<0.001) and ROIinternal (0.35±0.19 vs. 0.93±0.33, P<0.001), respectively, as illustrated in Figure 4A. For ratio of AUC, significantly lower values were revealed in responders on ROIwhole (0.35±0.21 vs. 0.86±0.26, P<0.001), ROIperipheral (0.28±0.19 vs. 0.86±0.24, P<0.001) and ROIinternal (0.36±0.20 vs. 0.89±0.26, P<0.001), respectively, as illustrated in Figure 4B. For ratio of MTT on ROIinternal, no significantly different values were revealed in responders as compared to non-responders (2.45±5.05 vs. 1.35±1.70, P=0.17).
Table 4
| Variables | Responders (n=48) | Non-responders (n=41) | P value | AUROC (95% CI) |
|---|---|---|---|---|
| Ratio of IMAX | ||||
| Whole lesion | 0.34±0.16 | 0.90±0.29 | <0.001 | 0.976 (0.919–0.997) |
| Lesion periphery | 0.29±0.20 | 0.88±0.36 | <0.001 | 0.964 (0.902–0.992) |
| Internal lesion | 0.35±0.19 | 0.93±0.33 | <0.001 | 0.966 (0.904–0.993) |
| Ratio of AUC | ||||
| Whole lesion | 0.35±0.21 | 0.86±0.26 | <0.001 | 0.938 (0.865–0.978) |
| Lesion periphery | 0.28±0.19 | 0.86±0.24 | <0.001 | 0.959 (0.895–0.990) |
| Internal lesion | 0.36±0.20 | 0.89±0.26 | <0.001 | 0.946 (0.876–0.983) |
| Ratio of MTT | ||||
| Internal lesion | 2.45±5.05 | 1.35±1.70 | 0.17 | 0.584 (0.475–0.688) |
Data are presented as mean ± standard deviation. CEUS, contrast-enhanced ultrasound; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; IMAX, maximal intensity; AUC, area under the time-intensity curve; MTT, mean transit time.
In discriminating responders from non-responders, poor performance of MTT ratio on ROIinternal was found with AUROC of 0.584 (0.475–0.688), while for ratios of IMAX and AUC, AUROCs from 0.938 to 0.976 were observed.
Reduction and ratio of CEUS perfusion parameters in assessing treatment response in development cohort
Diagnostic performances for reduction and ratio of CEUS perfusion parameters in assessing treatment response in development cohort based on mRECIST 2 months post-treatment are listed in Tables 5,6, respectively. For ∆IMAX, AUROCs of 0.942 (0.871–0.981), 0.939 (0.867–0.979), and 0.898 (0.816–0.952) were revealed on ROIwhole, ROIperipheral, and ROIinternal, respectively. For ∆AUC, AUROCs of 0.905 (0.824–0.957), 0.951 (0.883–0.985), and 0.885 (0.800–0.943) were revealed on ROIwhole, ROIperipheral, and ROIinternal, respectively (Figure 5).
Table 5
| Parameters | ROI | AUROC (95% CI) | Cut-off value | Responders, n | Non-responders, n | ACC (%) |
SEN (%) |
SPE (%) |
PPV (%) |
NPV (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Development cohort (PR vs. SD & PD: n=48 vs. n=41) | ||||||||||
| ∆IMAX | Whole lesion | 0.942 (0.871–0.981) | >21 | 44 | 6 | 89 | 92 | 85 | 88 | 90 |
| ≤21 | 4 | 35 | ||||||||
| Lesion periphery | 0.939 (0.867–0.979) | >44 | 41 | 2 | 90 | 85 | 95 | 95 | 85 | |
| ≤44 | 7 | 39 | ||||||||
| Internal lesion | 0.898 (0.816–0.952) | >22 | 34 | 3 | 81 | 71 | 93 | 92 | 73 | |
| ≤22 | 14 | 38 | ||||||||
| ∆AUC | Whole lesion | 0.905 (0.824–0.957) | >22 | 36 | 3 | 83 | 75 | 93 | 92 | 76 |
| ≤22 | 12 | 38 | ||||||||
| Lesion periphery | 0.951 (0.883–0.985) | >22 | 44 | 1 | 94 | 92 | 98 | 98 | 91 | |
| ≤22 | 4 | 40 | ||||||||
| Internal lesion | 0.885 (0.800–0.943) | >12 | 34 | 4 | 80 | 71 | 90 | 89 | 73 | |
| ≤12 | 14 | 37 | ||||||||
| Validation cohort (PR vs. SD & PD: n=14 vs. n=12) | ||||||||||
| ∆IMAX | Whole lesion | 0.815 (0.615–0.939) | >21 | 11 | 5 | 69 | 79 | 58 | 69 | 70 |
| ≤21 | 3 | 7 | ||||||||
| Lesion periphery | 0.917 (0.740–0.988) | >44 | 13 | 1 | 92 | 93 | 92 | 93 | 92 | |
| ≤44 | 1 | 11 | ||||||||
| Internal lesion | 0.732 (0.524–0.885) | >22 | 8 | 2 | 69 | 57 | 83 | 80 | 63 | |
| ≤22 | 6 | 10 | ||||||||
| ∆AUC | Whole lesion | 0.917 (0.740–0.988) | >22 | 12 | 1 | 88 | 86 | 92 | 92 | 85 |
| ≤22 | 2 | 11 | ||||||||
| Lesion periphery | 0.923 (0.748–0.990) | >22 | 14 | 1 | 96 | 100 | 92 | 93 | 100 | |
| ≤22 | 0 | 11 | ||||||||
| Internal lesion | 0.798 (0.595–0.928) | >12 | 8 | 2 | 69 | 57 | 83 | 80 | 63 | |
| ≤12 | 6 | 10 | ||||||||
Cut-offs maximizing the sum of sensitivity and specificity. ROI, region of interest; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; ACC, accuracy; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; PR, partial response; SD, stable disease; PD, progressive disease; ∆IMAX, IMAX of pre-treatment minus that of post-treatment; IMAX, maximal intensity; ∆AUC, AUC of pre-treatment minus that of post-treatment; AUC, area under the time-intensity curve.
Table 6
| Parameter | ROI | AUROC (95% CI) | Cut-off value | Responders, n | Non-responders, n | ACC (%) |
SEN (%) |
SPE (%) |
PPV (%) |
NPV (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Development cohort (PR vs. SD & PD: n=48 vs. n=41) | ||||||||||
| Ratio of IMAX | Whole lesion | 0.976 (0.919–0.997) | <0.58 | 46 | 3 | 94 | 96 | 93 | 94 | 95 |
| ≥0.58 | 2 | 38 | ||||||||
| Lesion periphery | 0.964 (0.902–0.992) | <0.57 | 45 | 2 | 94 | 94 | 95 | 96 | 93 | |
| ≥0.57 | 3 | 39 | ||||||||
| Internal lesion | 0.966 (0.904–0.993) | <0.56 | 45 | 3 | 93 | 94 | 93 | 94 | 93 | |
| ≥0.56 | 3 | 38 | ||||||||
| Ratio of AUC | Whole lesion | 0.938 (0.865–0.978) | <0.51 | 41 | 4 | 88 | 85 | 90 | 91 | 84 |
| ≥0.51 | 7 | 37 | ||||||||
| Lesion periphery | 0.959 (0.895–0.990) | <0.50 | 43 | 3 | 91 | 90 | 93 | 93 | 88 | |
| ≥0.50 | 5 | 38 | ||||||||
| Internal lesion | 0.946 (0.876–0.983) | <0.60 | 45 | 6 | 90 | 94 | 85 | 88 | 92 | |
| ≥0.60 | 3 | 35 | ||||||||
| Validation cohort (PR vs. SD & PD: n=14 vs. n=12) | ||||||||||
| Ratio of IMAX | Whole lesion | 0.899 (0.717–0.982) | <0.58 | 13 | 2 | 88 | 93 | 83 | 87 | 91 |
| ≥0.58 | 1 | 10 | ||||||||
| Lesion periphery | 0.923 (0.748–0.990) | <0.57 | 13 | 2 | 88 | 93 | 83 | 87 | 91 | |
| ≥0.57 | 1 | 10 | ||||||||
| Internal lesion | 0.899 (0.717–0.982) | <0.56 | 11 | 2 | 81 | 79 | 83 | 85 | 77 | |
| ≥0.56 | 3 | 10 | ||||||||
| Ratio of AUC | Whole lesion | 0.982 (0.836–1.000) | <0.51 | 13 | 1 | 92 | 93 | 92 | 93 | 92 |
| ≥0.51 | 1 | 11 | ||||||||
| Lesion periphery | 0.982 (0.836–1.000) | <0.50 | 14 | 1 | 96 | 100 | 92 | 93 | 100 | |
| ≥0.50 | 0 | 11 | ||||||||
| Internal lesion | 0.940 (0.773–0.995) | <0.60 | 12 | 1 | 88 | 86 | 92 | 92 | 85 | |
| ≥0.60 | 2 | 11 | ||||||||
Cut-offs maximizing the sum of sensitivity and specificity. ROI, region of interest; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; ACC, accuracy; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; PR, partial response; SD, stable disease; PD, progressive disease; IMAX, maximal intensity; AUC, area under the time-intensity curve.

Influence of ROI selection on ∆par. in discrimination of responders from non-responders was analyzed. The AUROC for ∆AUC on ROIperipheral demonstrated significantly higher value as compared to ∆IMAX on ROIinternal, ∆AUC on ROIwhole, and ∆AUC on ROIinternal (all P<0.05). Comparable, however slightly lower, AUROCs were observed for ∆IMAX on ROIwhole and ∆IMAX on ROIperipheral, as compared to ∆AUC on ROIperipheral (all P>0.05). With a cut-off value of 22, the accuracy, sensitivity, specificity, PPV and NPV for ∆AUC on ROIperipheral in assessing treatment response was 94%, 92%, 98%, 98% and 91%, respectively. With a cut-off value of 44, the accuracy, sensitivity, specificity, PPV and NPV for ∆IMAX on ROIperipheral in assessing treatment response was 90%, 85%, 95%, 95% and 85%, respectively.
As also illustrated in Table 6, in discrimination of responders from non-responders, for ratio of IMAX, AUROCs of 0.976 (0.919–0.997), 0.964 (0.902–0.992), and 0.966 (0.904–0.993) were revealed on ROIwhole, ROIperipheral, and ROIinternal, respectively. For ratio of AUC, AUROCs of 0.938 (0.865–0.978), 0.959 (0.895–0.990), and 0.946 (0.876–0.983) were revealed on ROIwhole, ROIperipheral, and ROIinternal, respectively. No influence of ROI selection was observed to discriminate responders from non-responders using the ratio of perfusion parameter, as no significantly different AUROCs were revealed (all P>0.05). The accuracy for ratio of IMAX ranged from 93% (ROIinternal) to 94% (ROIwhole and ROIperiphery), and accuracy for ratio of AUC ranged from 88% (ROIwhole) to 91%(ROIperipheral), respectively, in assessing treatment response.
Reduction and ratio of CEUS perfusion parameters in assessing treatment response in validation cohort
Diagnostic performances for ∆par. and ratio of parameters in assessing treatment response in validation cohort are demonstrated in Tables 5,6, respectively. According to mRECIST, the validation cohort included 14 responders (14 PR) and 12 non-responders (10 SD, 2 PD). Similar as in the development cohort, influence of ROI selection was observed when using ∆par. Significantly higher AUROC was observed for ∆AUC on ROIperipheral as compared to ∆IMAX on ROIwhole, ∆IMAX on ROIinternal, and ∆AUC on ROIinternal (AUROCs: 0.923 vs. 0.815 & 0.732 & 0.798, all P<0.05). ∆IMAX on ROIperipheral had comparable AUROC as compared to ∆AUC on ROIperipheral and ∆IMAX on ROIperipheral (AUROC: 0.923 vs. 0.917 vs. 0.917, all P>0.05). With the cut-offs derived from the development cohort, the accuracy, sensitivity, specificity, PPV, and NPV were 96%, 100%, 92%, 93% and 100% for ∆AUC on ROIperipheral and were 92%, 93%, 92%, 93% and 92% for ∆IMAX on ROIperipheral (Figure 6).

Regarding to the ratio of perfusion parameters in validation cohort, as illustrated in the development cohort, no influence of ROI selection was observed in assessing treatment response, as comparable AUROCs were observed on all parameters with AUROCs ranging from 0.899 to 0.982 with all P>0.05. Using the cut-offs derived from the development cohort, reductions of 6–12% in accuracy were observed for ratios of IMAX in validation cohort. Differences of −4% to 5% in accuracy were observed in the validation cohort as compared to development cohort when using ratios of AUC.
Discussion
Currently, imaging biomarkers for response assessment of liver tumors under treatment have been investigated using various functional imaging modalities, such as DCE-MRI, DCE-CT, PET/CT, and CEUS (19-22). DCE-MRI has a major problem of the conflict between spatial resolution and scanning rate for dynamic tracking; while potential renal toxicity of CT contrast agent together with radiation limit its utilization for frequent response assessment. Except for the exposure of ionizing radiation, PET/CT also suffers from low spatial resolution. With the advantages of real-time imaging and radiation-free, CEUS has received more investigation for the assessment and monitor of treatment response in various tumors nowadays, and promising results have been reported (23-25). Due to the pure blood pool nature of ultrasound contrast agent (SonoVue), CEUS perfusion parameters can quantitatively reflect the microvascularity within different organs and lesions. However, few research concerning CEUS quantitative analysis has ever been conducted on patients with CRLM. As an amendment of RECIST, mRECIST was introduced in 2008, and focused on the viable (enhancing on dynamic modalities) tumoral components to assess tumor response. It is based on the uni-dimensional measurement on CECT or DCE-MRI, which are the standard modalities for an imaging assessment of tumor response (15). There is growing evidence that mRECIST is a valuable prognostic tool to predict overall and disease-free survival in patients with liver tumors (26,27). Therefore, in present study, with the treatment response graded by mRECIST as reference, we comprehensively studied the utility of CEUS perfusion parameters in assessing treatment response in patients with CRLM for chemotherapy with Bev.
In present study, to comprehensively study the correlation between tumor blood perfusion and treatment response, three different analysis ROIs were selected on the whole lesion, lesion periphery, and lesion internal, respectively, on each study subject. In the development cohort, significant ∆IMAX and ∆AUC in responders (n=48) were observed on all three analysis ROIs, while comparable post- and pre-treatment values of IMAX and AUC were found on all ROIs in non-responders (n=41). The significant ∆IMAX and ∆AUC in responders were induced by the synergistic anti-angiogenetic effect of Bev and cytotoxic therapy. As a monoclonal antibody against VEGF, Bev might reduce the tumor vascularity which could be revealed by the reduction of blood volume within the tumor. Some of the CEUS perfusion parameters, such as IMAX and AUC, were related to the tumor blood volume. Thus, they may be predictive biomarkers in the assessment of treatment response. As reported in Lassau’s study on the treatment response assessment of HCC to Bev, the reduction of contrast agent uptake in tumor correlated well with CEUS perfusion parameters, and ∆AUC showed correlation with RECIST response (24). While, in the study of Ramin on 30 patients with CRLMs for FOLFIRI plus Bev treatment, ∆IMAX between pre- and post-treatment on responders (n=13) was the same as that on non-responders (n=17) (28). One reason to induce these discrepancies between different studies might be that angiogenesis is a complex process and can be influenced by many local and systemic factors. This fact might introduce variability in the treatment response among different tumor types and even within the individuals with the same tumor type. Therefore, large cohort of study subjects should be included. Another reason might be that different ROI selection protocols were utilized cross different studies.
In present study, we did find the influence of ROI selection on the assessment of treatment response using ∆par. As revealed in the development cohort and validated in the validation cohort, ∆IMAX and ∆AUC on ROIperipheral significantly outperformed other ∆par. in assessing treatment response, with high AUROCs (0.939–0.951 and 0.917–0.923, respectively for two cohorts) and accuracies (90–94% and 92–96%, respectively for two cohorts). ∆IMAX and ∆AUC on ROIwhole performed secondly with relative lower AUROCs (0.905–0.942 and 0.815–0.917, respectively in two cohorts) and accuracies (83–89% and 69–88%, respectively in two cohorts). ∆IMAX and ∆AUC on ROIinternal performed the worst with the lowest AUROCs (0.885–0.898 and 0.732–0.798, respectively in two cohorts) and accuracies (80–81% and 69%, respectively in two cohorts). This might be explained by the histological characteristics of CRLM. The tumor blood vessels of CRLM are structurally abnormal, resulting in heterogeneity of blood flow throughout the tumor, with tumor periphery more vascular, relatively (29,30). Furthermore, with the common invasive growth pattern of malignancy, tumor periphery is also regarded as necrosis irrelevant. From pathological perspective, the treatment response of chemotherapy with Bev to CRLM is associated with decrease of residual tumor cells and micro-vascular density, together with increase of necrosis and fibrotic involution (31). In studies concerning the pathological changes occurring in the CRLM after chemotherapy, the presence of residual cells and tumor angiogenesis was reported to be related to the contrast effect of the peripheral tumor margin on CECT (15,32). As tumor periphery is regarded as the most vascularized area within the tumor, characterized by numerous immature blood vessels, ROIs selected on tumor periphery should be more representative of tumor vascularity. Therefore, the changes of perfusion parameters on ROIperipheral during treatment should theoretically be more sensitive in assessing tumor response. By revealing the blood perfusion and necrosis of whole lesion, parameters calculated from ROIwhole should also be useful indicators in assessing treatment response, which also showed good performance (AUROC: 0.905–0.976). In our study, all the patients were evaluated at 2 months after treatment, and no patients had CR. Therefore, more patients with longer periods of treatment should be included in further study.
Regarding to the ratio of perfusion parameters, no influence of ROI selection on the response assessment was found. In present study, ratios of IMAX and AUC on all ROIs demonstrated high AUROCs in both development cohort (AUROCs: 0.938–0.976) and validation cohort (AUROCs: 0.899–0.982). Similar findings have been reported on the response evaluation of different tumors under different targeted anti-angiogenetic therapies (24,33). In present study, compared with ∆IMAX and ∆AUC from different analysis ROIs, the ratio of corresponding parameter on different ROIs showed higher AUROCs. This may be accounted by the vascular heterogeneity among different individuals, even with the same disease, which may induce wide range of IMAX and AUC in CRLM pre-treatment. Therefore, during perfusion analysis, the calculation of ratio of parameters can partly minimize these variations by normalizing the target measurement, to some extent, compared with that of ∆par. However, using the same cut-offs derived from the development cohort, decrease in accuracy (>5%) was observed for ratios of IMAX and AUC in validation cohort. While for ∆IMAX and ∆AUC on ROIperipheral, using the same cut-offs, similar accuracy was revealed in the two cohorts. Therefore, multi-center study with more patients should be performed to further study the clinical utilities of perfusion parameters (IMAX and AUC) in assessing treatment response to chemotherapy with Bev on CRLM patients.
Our study is unique from several aspects. Firstly, we included two independent cohorts of study subjects: the development cohort (n=89) to reveal the valuable CEUS perfusion parameters for tumor response assessment, and the validation cohort (n=26) to further verify the clinical value of these significant parameters. Secondly, reductions and ratios of parameters post- and pre-treatment were comprehensively studied from three different analysis analysis ROIs. Moreover, the influence of ROI selection on the assessment of treatment response using CEUS perfusion parameters was investigated. However, there are still some limitations in our study. Firstly, the study cohort was relatively small and the period for follow-up was not long enough to study the progression free survival and overall survival. More patients with long-term follow-up would be involved in the future. Secondly, only one target lesion per patient was investigated in this study. It would be of interest to include all liver metastases of one patient to further assess the power of this method.
Conclusions
IMAX and AUC showed significant reductions in responders, and different ROIs within the tumor influence the performance of ∆IMAX and ∆AUC in response assessment. Parameters derived from ROIperipheral exhibited the most promising results in predicting treatment response.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1027/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1027/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This prospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Zhongshan Hospital, Fudan University approved the study (No. B2021-347R), and written informed consent was received from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Cardona K, Mastrodomenico P, D'Amico F, Shia J, Gönen M, Weiser MR, Paty PB, Kingham TP, Allen PJ, De Matteo RP, Fong Y, Jarnagin WR, D'Angelica MI. Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol 2013;20:148-54. [Crossref] [PubMed]
- Imai K, Allard MA, Benitez CC, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist 2016;21:887-94. [Crossref] [PubMed]
- Edwards MS, Chadda SD, Zhao Z, Barber BL, Sykes DP. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis 2012;14:e31-47. [Crossref] [PubMed]
- Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 2007;74:72-84. [Crossref] [PubMed]
- Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007;25:4779-86. [Crossref] [PubMed]
- Pectasides D, Papaxoinis G, Kalogeras KT, Eleftheraki AG, Xanthakis I, Makatsoris T, Samantas E, Varthalitis I, Papakostas P, Nikitas N, Papandreou CN, Pentheroudakis G, Timotheadou E, Koutras A, Sgouros J, Bafaloukos D, Klouvas G, Economopoulos T, Syrigos KN, Fountzilas G. XELIRI-bevacizumab versus FOLFIRI-bevacizumab as first-line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology Group phase III trial with collateral biomarker analysis. BMC Cancer 2012;12:271. [Crossref] [PubMed]
- Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:141-52; quiz 152. [Crossref] [PubMed]
- Padhani AR, Ollivier L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: implications for diagnostic radiologists. Br J Radiol 2001;74:983-6. [Crossref] [PubMed]
- Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 2009;115:616-23. [Crossref] [PubMed]
- Primavesi F, Fadinger N, Biggel S, Braunwarth E, Gasser E, Sprung S, Göbel G, Gassner E, Stättner S, Öfner D. Early response evaluation during preoperative chemotherapy for colorectal liver metastases: Combined size and morphology-based criteria predict pathological response and survival after resection. J Surg Oncol 2020;121:382-91. [Crossref] [PubMed]
- Cheng J, Qiu M, Zhang Y, Hong N, Shen D, Zhou J, Wang Y. Enhanced Rim on MDCT of Colorectal Liver Metastases: Assessment of Ability to Predict Progression-Free Survival and Response to Bevacizumab-Based Chemotherapy. AJR Am J Roentgenol 2020;215:1377-83. [Crossref] [PubMed]
- Burger IA, Schwarz EI, Samarin A, Breitenstein S, Weber A, Hany TF. Correlation between therapy response assessment using FDG PET/CT and histopathologic tumor regression grade in hepatic metastasis of colorectal carcinoma after neoadjuvant therapy. Ann Nucl Med 2013;27:177-83. [Crossref] [PubMed]
- De Bruyne S, Van Damme N, Smeets P, Ferdinande L, Ceelen W, Mertens J, Van de Wiele C, Troisi R, Libbrecht L, Laurent S, Geboes K, Peeters M. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer 2012;106:1926-33. [Crossref] [PubMed]
- Ishida K, Tamura A, Kato K, Uesugi N, Osakabe M, Eizuka M, Hasegawa Y, Nitta H, Otsuka K, Sasaki A, Ehara S, Sugai T. Correlation between CT morphologic appearance and histologic findings in colorectal liver metastasis after preoperative chemotherapy. Abdom Radiol (NY) 2018;43:2991-3000. [Crossref] [PubMed]
- Xin Y, Xiong Y, Liu F, Qu L, Li W, Yang L, Liu Y, Zhu J. Quantitative analysis of contrast-enhanced ultrasonography in rat models of hepatic acute graft-versus-host disease. Quant Imaging Med Surg 2023;13:4908-18. [Crossref] [PubMed]
- Kratzer W, Güthle M, Dobler F, Seufferlein T, Graeter T, Schmidberger J, Barth TF, Klaus J. Comparison of superb microvascular imaging (SMI) quantified with ImageJ to quantified contrast-enhanced ultrasound (qCEUS) in liver metastases-a pilot study. Quant Imaging Med Surg 2022;12:1762-74. [Crossref] [PubMed]
- Lu Q, Huang BJ, Xue LY, Fan PL, Wang WP. Differentiation of Renal Tumor Histotypes: Usefulness of Quantitative Analysis of Contrast-Enhanced Ultrasound. AJR Am J Roentgenol 2015;205:W335-42. [Crossref] [PubMed]
- García Vicente AM, Domínguez Ferreras E, Sánchez Pérez V, Poblete García VM, Villa Guzmán JC, Jiménez Aragón F, Pineda Pineda MD, Molino Trinidad C, Soriano Castrejón Á. Response assessment of colorectal liver metastases with contrast enhanced CT/18F-FDG PET. Eur J Radiol 2013;82:e255-61. [Crossref] [PubMed]
- Mazard T, Boonsirikamchai P, Overman MJ, Asran MA, Choi H, Herron D, Eng C, Maru DM, Ychou M, Vauthey JN, Loyer EM, Kopetz S. Comparison of early radiological predictors of outcome in patients with colorectal cancer with unresectable hepatic metastases treated with bevacizumab. Gut 2018;67:1095-102. [Crossref] [PubMed]
- Engelmann BE, Loft A, Kjær A, Nielsen HJ, Gerds TA, Benzon EV, Brünner N, Christensen IJ, Hansson SH, Holländer NH, Kristensen MH, Löfgren J, Markova E, Sloth C, Højgaard L. Positron emission tomography/computed tomography and biomarkers for early treatment response evaluation in metastatic colon cancer. Oncologist 2014;19:164-72. [Crossref] [PubMed]
- Heijmen L, Verstappen MC, Ter Voert EE, Punt CJ, Oyen WJ, de Geus-Oei LF, Hermans JJ, Heerschap A, van Laarhoven HW. Tumour response prediction by diffusion-weighted MR imaging: ready for clinical use? Crit Rev Oncol Hematol 2012;83:194-207. [Crossref] [PubMed]
- Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013;39:187-210. [Crossref] [PubMed]
- Lassau N, Koscielny S, Chami L, Chebil M, Benatsou B, Roche A, Ducreux M, Malka D, Boige V. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification--preliminary results. Radiology 2011;258:291-300. [Crossref] [PubMed]
- Lassau N, Lamuraglia M, Chami L, Leclère J, Bonvalot S, Terrier P, Roche A, Le Cesne A. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol 2006;187:1267-73. [Crossref] [PubMed]
- Kim BK, Kim KA, Park JY, Ahn SH, Chon CY, Han KH, Kim SU, Kim MJ. Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation. Eur J Cancer 2013;49:826-34. [Crossref] [PubMed]
- Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 2012;118:147-56. [Crossref] [PubMed]
- Schirin-Sokhan R, Winograd R, Roderburg C, Bubenzer J. do Ó NC, Guggenberger D, Hecker H, Trautwein C, Tischendorf JJ. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J Gastroenterol 2012;18:541-5. [Crossref] [PubMed]
- Masuishi T, Taniguchi H, Eto T, Komori A, Mitani S, Hasegawa H, Narita Y, Ishihara M, Tanaka T, Kadowaki S, Ura T, Ando M, Tajika M, Nomura M, Sato Y, Mishima H, Muro K. Morphologic Response and Tumor Shrinkage as Early Predictive Markers in Unresectable Colorectal Liver Metastases. Anticancer Res 2018;38:6501-6. [Crossref] [PubMed]
- Hegenscheid K, Behrendt N, Rosenberg C, Kuehn JP, Ewert R, Hosten N, Puls R. Assessing early vascular changes and treatment response after laser-induced thermotherapy of pulmonary metastases with perfusion CT: initial experience. AJR Am J Roentgenol 2010;194:1116-23. [Crossref] [PubMed]
- Sebagh M, Allard MA, Cunha AS, Ruiz A, Araujo R, Lemoine A, Paule B, Delvart V, Cherqui D, Vibert E, Adam R. A proposed new method for assessing the pathological response to chemotherapy in resected colorectal liver metastases. Br J Cancer 2014;111:470-6. [Crossref] [PubMed]
- Tamura A, Ishida K, Sone M, Yoshioka K. Evaluation of peripheral enhancement on contrast-enhanced computed tomography and corresponding pathological findings in colorectal liver metastases after preoperative chemotherapy. Pol J Radiol 2023;88:e251-5. [Crossref] [PubMed]
- Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol 2014;49:794-800. [Crossref] [PubMed]


